Abstract
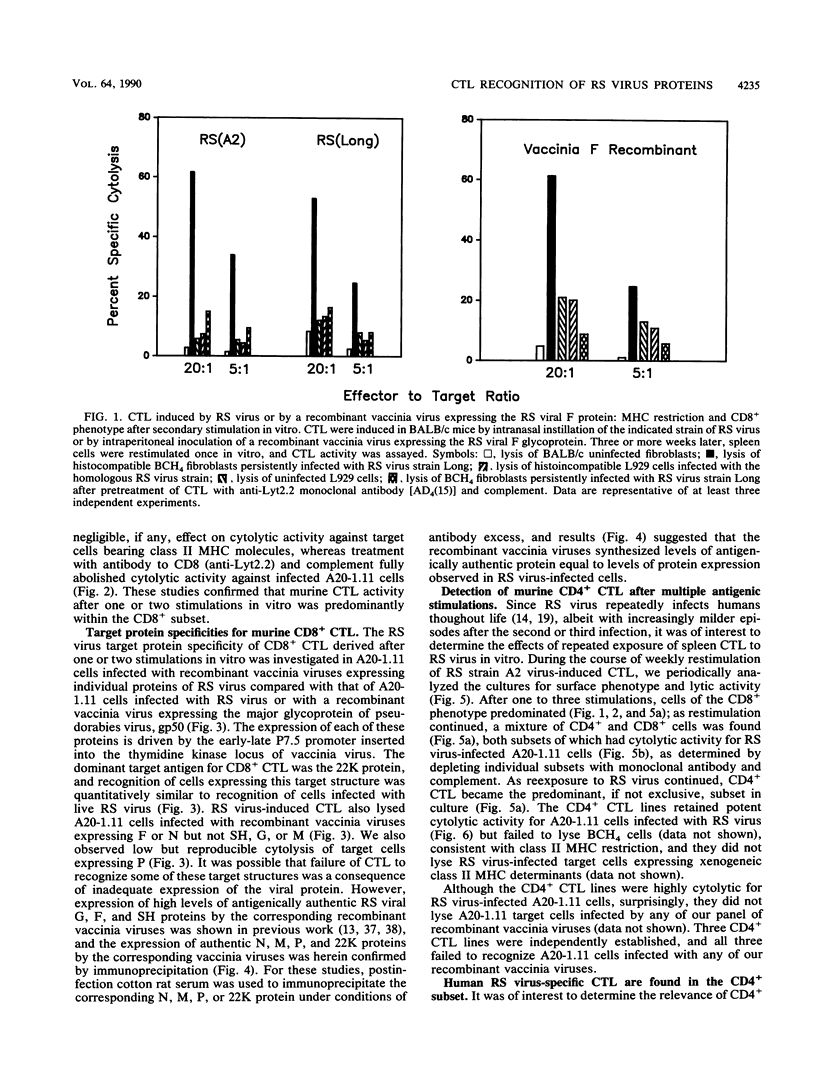
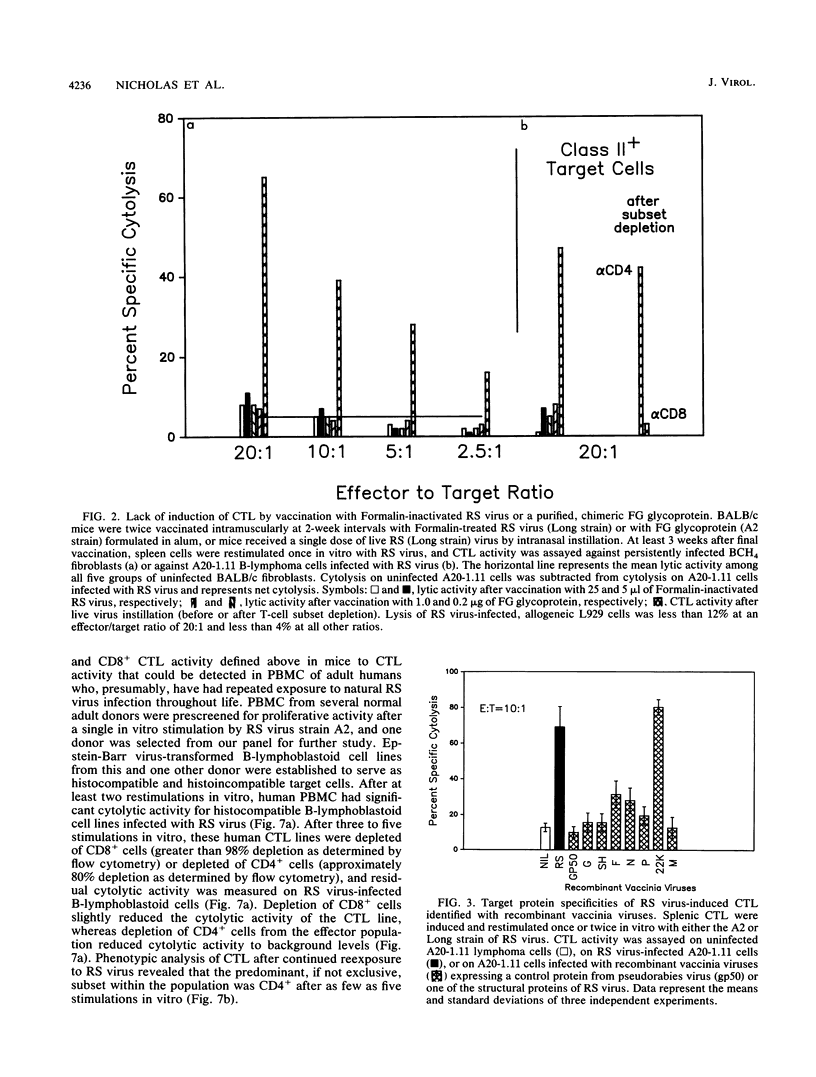
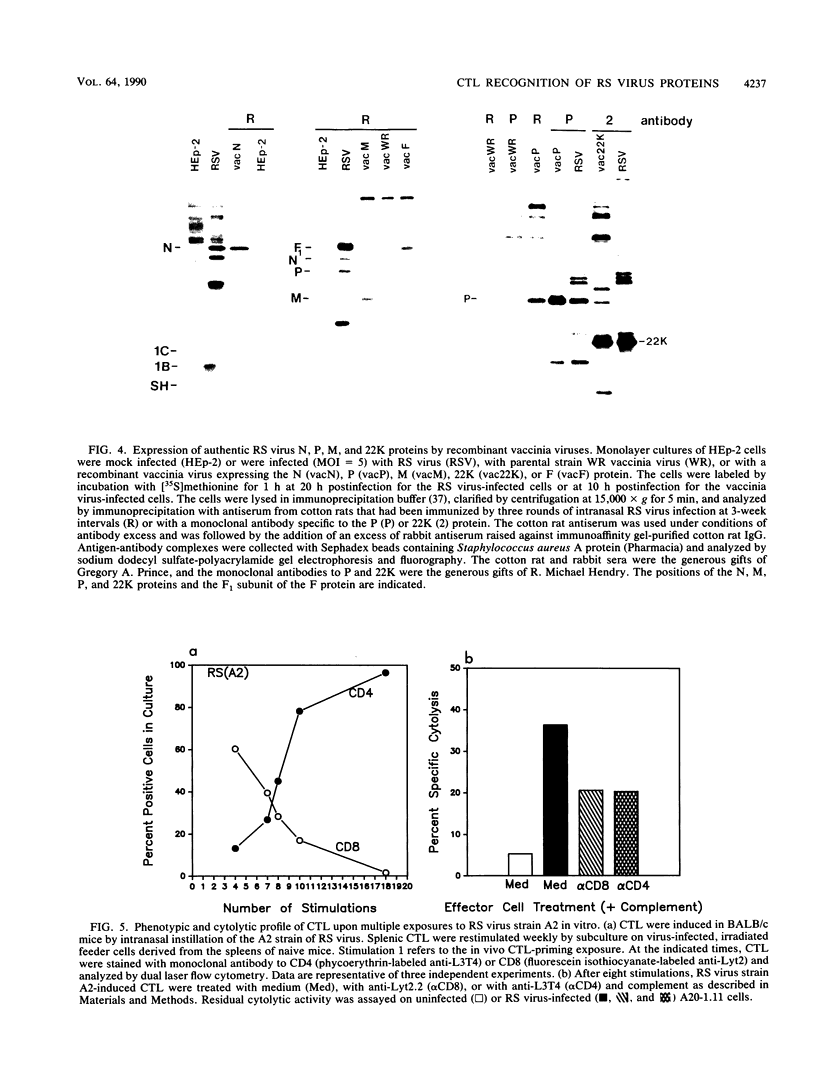
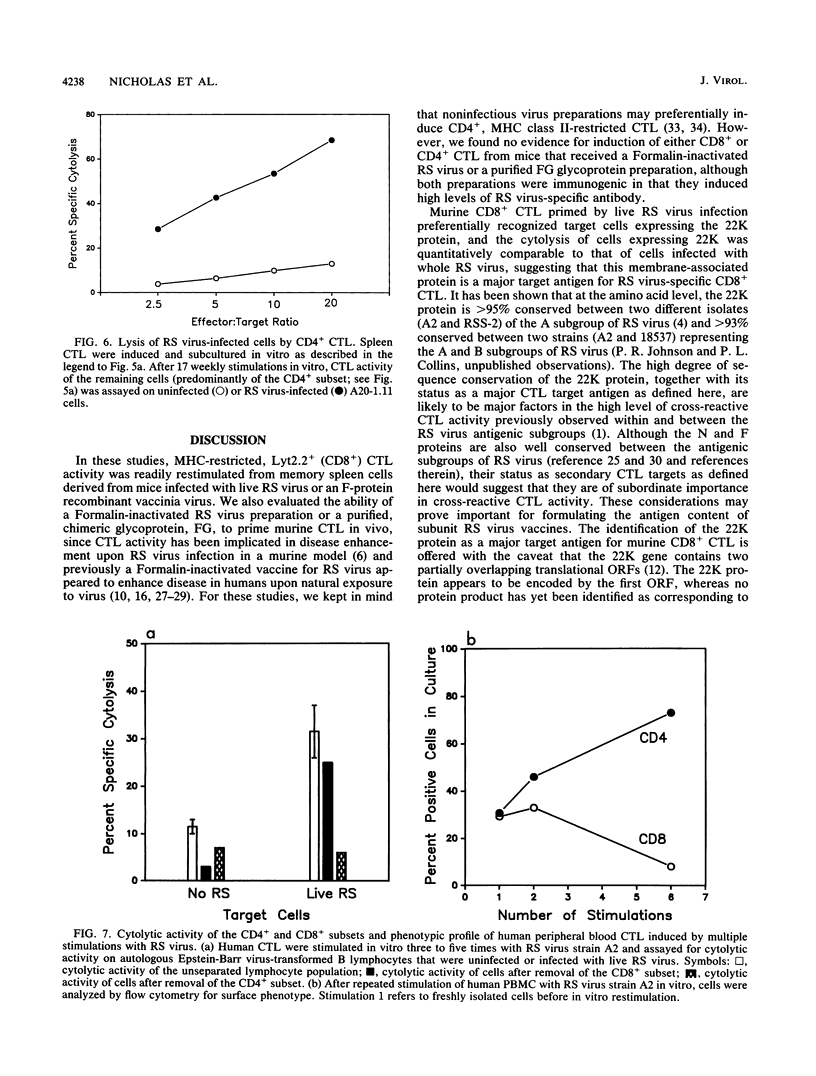
Cytolytic T-lymphocyte (CTL) activity specific for respiratory syncytial (RS) virus was investigated after intranasal infection of mice with RS virus, after intraperitoneal infection of mice with a recombinant vaccinia virus expressing the F glycoprotein, and after intramuscular vaccination of mice with Formalin-inactivated RS virus or a chimeric glycoprotein, FG, expressed from a recombinant baculovirus. Spleen cell cultures from mice previously infected with live RS virus or the F-protein recombinant vaccinia virus had significant CTL activity after one cycle of in vitro restimulation with RS virus, and lytic activity was derived from a major histocompatibility complex-restricted, Lyt2.2+ (CD8+) subset. CTL activity was not restimulated in spleen cells from mice that received either the Formalin-inactivated RS virus or the purified glycoprotein, FG. The protein target structures for recognition by murine CD8+ CTL were identified by using target cells infected with recombinant vaccinia viruses that individually express seven structural proteins of RS virus. Quantitation of cytolytic activity against cells expressing each target structure suggested that 22K was the major target protein for CD8+ CTL, equivalent to recognition of cells infected with RS virus, followed by intermediate recognition of F or N, slight recognition of P, and no recognition of G, SH, or M. Repeated stimulation of murine CTL with RS virus resulted in outgrowth of CD4+ CTL which, over time, became the exclusive subset in culture. Murine CD4+ CTL were highly cytolytic for RS virus-infected cells, but they did not recognize target cells infected with any of the recombinant vaccinia viruses expressing the seven RS virus structural proteins. Finally, the CTL response in peripheral blood mononuclear cells of adult human volunteers was investigated. The detection of significant levels of RS virus-specific cytolytic activity in these cells was dependent on at least two restimulations with RS virus in vitro, and cytolytic activity was derived primarily from the CD4+ subset.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bangham C. R., Askonas B. A. Murine cytotoxic T cells specific to respiratory syncytial virus recognize different antigenic subtypes of the virus. J Gen Virol. 1986 Apr;67(Pt 4):623–629. doi: 10.1099/0022-1317-67-4-623. [DOI] [PubMed] [Google Scholar]
- Bangham C. R., McMichael A. J. Specific human cytotoxic T cells recognize B-cell lines persistently infected with respiratory syncytial virus. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9183–9187. doi: 10.1073/pnas.83.23.9183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangham C. R., Openshaw P. J., Ball L. A., King A. M., Wertz G. W., Askonas B. A. Human and murine cytotoxic T cells specific to respiratory syncytial virus recognize the viral nucleoprotein (N), but not the major glycoprotein (G), expressed by vaccinia virus recombinants. J Immunol. 1986 Dec 15;137(12):3973–3977. [PubMed] [Google Scholar]
- Baybutt H. N., Pringle C. R. Molecular cloning and sequencing of the F and 22K membrane protein genes of the RSS-2 strain of respiratory syncytial virus. J Gen Virol. 1987 Nov;68(Pt 11):2789–2796. doi: 10.1099/0022-1317-68-11-2789. [DOI] [PubMed] [Google Scholar]
- Brideau R. J., Walters R. R., Stier M. A., Wathen M. W. Protection of cotton rats against human respiratory syncytial virus by vaccination with a novel chimeric FG glycoprotein. J Gen Virol. 1989 Oct;70(Pt 10):2637–2644. doi: 10.1099/0022-1317-70-10-2637. [DOI] [PubMed] [Google Scholar]
- Cannon M. J., Openshaw P. J., Askonas B. A. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med. 1988 Sep 1;168(3):1163–1168. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba Y., Higashidate Y., Suga K., Honjo K., Tsutsumi H., Ogra P. L. Development of cell-mediated cytotoxic immunity to respiratory syncytial virus in human infants following naturally acquired infection. J Med Virol. 1989 Jul;28(3):133–139. doi: 10.1002/jmv.1890280304. [DOI] [PubMed] [Google Scholar]
- Chin J., Magoffin R. L., Shearer L. A., Schieble J. H., Lennette E. H. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969 Apr;89(4):449–463. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- Collins P. L., Anderson K., Langer S. J., Wertz G. W. Correct sequence for the major nucleocapsid protein mRNA of respiratory syncytial virus. Virology. 1985 Oct 15;146(1):69–77. doi: 10.1016/0042-6822(85)90053-4. [DOI] [PubMed] [Google Scholar]
- Collins P. L., Wertz G. W. The envelope-associated 22K protein of human respiratory syncytial virus: nucleotide sequence of the mRNA and a related polytranscript. J Virol. 1985 Apr;54(1):65–71. doi: 10.1128/jvi.54.1.65-71.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elango N., Prince G. A., Murphy B. R., Venkatesan S., Chanock R. M., Moss B. Resistance to human respiratory syncytial virus (RSV) infection induced by immunization of cotton rats with a recombinant vaccinia virus expressing the RSV G glycoprotein. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1906–1910. doi: 10.1073/pnas.83.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald G. W., Almond J. R., Henderson F. W. Cellular and humoral immunity in recurrent respiratory syncytial virus infections. Pediatr Res. 1983 Sep;17(9):753–758. doi: 10.1203/00006450-198309000-00014. [DOI] [PubMed] [Google Scholar]
- Fernie B. F., Ford E. C., Gerin J. L. The development of Balb/c cells persistently infected with respiratory syncytial virus: presence of ribonucleoprotein on the cell surface. Proc Soc Exp Biol Med. 1981 May;167(1):83–86. doi: 10.3181/00379727-167-41129. [DOI] [PubMed] [Google Scholar]
- Fulginiti V. A., Eller J. J., Sieber O. F., Joyner J. W., Minamitani M., Meiklejohn G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol. 1969 Apr;89(4):435–448. doi: 10.1093/oxfordjournals.aje.a120956. [DOI] [PubMed] [Google Scholar]
- Glezen W. P., Taber L. H., Frank A. L., Kasel J. A. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986 Jun;140(6):543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- Halloran P. F., Wadgymar A., Autenried P. The regulation of expression of major histocompatibility complex products. Transplantation. 1986 Apr;41(4):413–420. [PubMed] [Google Scholar]
- Henderson F. W., Collier A. M., Clyde W. A., Jr, Denny F. W. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med. 1979 Mar 8;300(10):530–534. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- Huang Y. T., Collins P. L., Wertz G. W. Characterization of the 10 proteins of human respiratory syncytial virus: identification of a fourth envelope-associated protein. Virus Res. 1985 Mar;2(2):157–173. doi: 10.1016/0168-1702(85)90246-1. [DOI] [PubMed] [Google Scholar]
- Isaacs D., Bangham C. R., McMichael A. J. Cell-mediated cytotoxic response to respiratory syncytial virus in infants with bronchiolitis. Lancet. 1987 Oct 3;2(8562):769–771. doi: 10.1016/s0140-6736(87)92502-5. [DOI] [PubMed] [Google Scholar]
- Issekutz T., Chu E., Geha R. S. Antigen presentation by human B cells: T cell proliferation induced by Epstein Barr virus B lymphoblastoid cells. J Immunol. 1982 Oct;129(4):1446–1450. [PubMed] [Google Scholar]
- Jacobson S., Sekaly R. P., Jacobson C. L., McFarland H. F., Long E. O. HLA class II-restricted presentation of cytoplasmic measles virus antigens to cytotoxic T cells. J Virol. 1989 Apr;63(4):1756–1762. doi: 10.1128/jvi.63.4.1756-1762.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. R., Collins P. L. The 1B (NS2), 1C (NS1) and N proteins of human respiratory syncytial virus (RSV) of antigenic subgroups A and B: sequence conservation and divergence within RSV genomic RNA. J Gen Virol. 1989 Jun;70(Pt 6):1539–1547. doi: 10.1099/0022-1317-70-6-1539. [DOI] [PubMed] [Google Scholar]
- Kallenberg C. G., Schilizzi B. M., Beaumont F., Poppema S., De Leij L., The T. H. Expression of class II MHC antigens on alveolar epithelium in fibrosing alveolitis. Clin Exp Immunol. 1987 Jan;67(1):182–190. [PMC free article] [PubMed] [Google Scholar]
- Kapikian A. Z., Mitchell R. H., Chanock R. M., Shvedoff R. A., Stewart C. E. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969 Apr;89(4):405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Canchola J. G., Brandt C. D., Pyles G., Chanock R. M., Jensen K., Parrott R. H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969 Apr;89(4):422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Leikin S. L., Arrobio J., Brandt C. D., Chanock R. M., Parrott R. H. Cell-mediated immunity to respiratory syncytial virus induced by inactivated vaccine or by infection. Pediatr Res. 1976 Jan;10(1):75–78. doi: 10.1203/00006450-197601000-00015. [DOI] [PubMed] [Google Scholar]
- López J. A., Villanueva N., Melero J. A., Portela A. Nucleotide sequence of the fusion and phosphoprotein genes of human respiratory syncytial (RS) virus Long strain: evidence of subtype genetic heterogeneity. Virus Res. 1988 May;10(2-3):249–261. doi: 10.1016/0168-1702(88)90020-2. [DOI] [PubMed] [Google Scholar]
- Marchioli C. C., Yancey R. J., Jr, Petrovskis E. A., Timmins J. G., Post L. E. Evaluation of pseudorabies virus glycoprotein gp50 as a vaccine for Aujeszky's disease in mice and swine: expression by vaccinia virus and Chinese hamster ovary cells. J Virol. 1987 Dec;61(12):3977–3982. doi: 10.1128/jvi.61.12.3977-3982.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer D. H., Hanke J. H., Mickelson E., Rich R. R., Pollack M. S. Differential presentation of HLA-DR, DQ, and DP restriction elements by interferon-gamma-treated dermal fibroblasts. J Immunol. 1987 Aug 1;139(3):715–723. [PubMed] [Google Scholar]
- Moore M. W., Carbone F. R., Bevan M. J. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988 Sep 9;54(6):777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- Morrison L. A., Braciale V. L., Braciale T. J. Antigen form influences induction and frequency of influenza-specific class I and class II MHC-restricted cytolytic T lymphocytes. J Immunol. 1988 Jul 15;141(2):363–368. [PubMed] [Google Scholar]
- Morrison L. A., Lukacher A. E., Braciale V. L., Fan D. P., Braciale T. J. Differences in antigen presentation to MHC class I-and class II-restricted influenza virus-specific cytolytic T lymphocyte clones. J Exp Med. 1986 Apr 1;163(4):903–921. doi: 10.1084/jem.163.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Sotnikov A., Paradiso P. R., Hildreth S. W., Jenson A. B., Baggs R. B., Lawrence L., Zubak J. J., Chanock R. M., Beeler J. A. Immunization of cotton rats with the fusion (F) and large (G) glycoproteins of respiratory syncytial virus (RSV) protects against RSV challenge without potentiating RSV disease. Vaccine. 1989 Dec;7(6):533–540. doi: 10.1016/0264-410x(89)90278-8. [DOI] [PubMed] [Google Scholar]
- Nicholas J. A., Levely M. E., Brideau R. J., Berger A. E. During recovery from cytomegalovirus infection T-lymphocyte subsets become selectively responsive to activation and have depressed interleukin 2 (IL2) secretion and IL2 receptor expression. Microb Pathog. 1987 Jan;2(1):37–47. doi: 10.1016/0882-4010(87)90113-6. [DOI] [PubMed] [Google Scholar]
- Olmsted R. A., Collins P. L. The 1A protein of respiratory syncytial virus is an integral membrane protein present as multiple, structurally distinct species. J Virol. 1989 May;63(5):2019–2029. doi: 10.1128/jvi.63.5.2019-2029.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted R. A., Elango N., Prince G. A., Murphy B. R., Johnson P. R., Moss B., Chanock R. M., Collins P. L. Expression of the F glycoprotein of respiratory syncytial virus by a recombinant vaccinia virus: comparison of the individual contributions of the F and G glycoproteins to host immunity. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7462–7466. doi: 10.1073/pnas.83.19.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Openshaw P. J., Anderson K., Wertz G. W., Askonas B. A. The 22,000-kilodalton protein of respiratory syncytial virus is a major target for Kd-restricted cytotoxic T lymphocytes from mice primed by infection. J Virol. 1990 Apr;64(4):1683–1689. doi: 10.1128/jvi.64.4.1683-1689.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton R. M., Cannon M. J., Openshaw P. J., Ball L. A., Wertz G. W., Askonas B. A. Cytotoxic T cell specificity for respiratory syncytial virus proteins: fusion protein is an important target antigen. J Gen Virol. 1987 Aug;68(Pt 8):2177–2182. doi: 10.1099/0022-1317-68-8-2177. [DOI] [PubMed] [Google Scholar]
- Prince G. A., Jenson A. B., Hemming V. G., Murphy B. R., Walsh E. E., Horswood R. L., Chanock R. M. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by prior intramuscular inoculation of formalin-inactiva ted virus. J Virol. 1986 Mar;57(3):721–728. doi: 10.1128/jvi.57.3.721-728.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puddington L., Bevan M. J., Rose J. K., Lefrançois L. N protein is the predominant antigen recognized by vesicular stomatitis virus-specific cytotoxic T cells. J Virol. 1986 Nov;60(2):708–717. doi: 10.1128/jvi.60.2.708-717.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake M., Elango N., Venkatesan S. Sequence analysis of the respiratory syncytial virus phosphoprotein gene. J Virol. 1984 Dec;52(3):991–994. doi: 10.1128/jvi.52.3.991-994.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake M., Venkatesan S. Nucleotide sequence of the gene encoding respiratory syncytial virus matrix protein. J Virol. 1984 Apr;50(1):92–99. doi: 10.1128/jvi.50.1.92-99.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G., Stott E. J., Hayle A. J. Cytotoxic lymphocytes in the lungs of mice infected with respiratory syncytial virus. J Gen Virol. 1985 Dec;66(Pt 12):2533–2538. doi: 10.1099/0022-1317-66-12-2533. [DOI] [PubMed] [Google Scholar]
- Wathen M. W., Brideau R. J., Thomsen D. R., Murphy B. R. Characterization of a novel human respiratory syncytial virus chimeric FG glycoprotein expressed using a baculovirus vector. J Gen Virol. 1989 Oct;70(Pt 10):2625–2635. doi: 10.1099/0022-1317-70-10-2625. [DOI] [PubMed] [Google Scholar]
- van Binnendijk R. S., Poelen M. C., de Vries P., Voorma H. O., Osterhaus A. D., Uytdehaag F. G. Measles virus-specific human T cell clones. Characterization of specificity and function of CD4+ helper/cytotoxic and CD8+ cytotoxic T cell clones. J Immunol. 1989 Apr 15;142(8):2847–2854. [PubMed] [Google Scholar]



