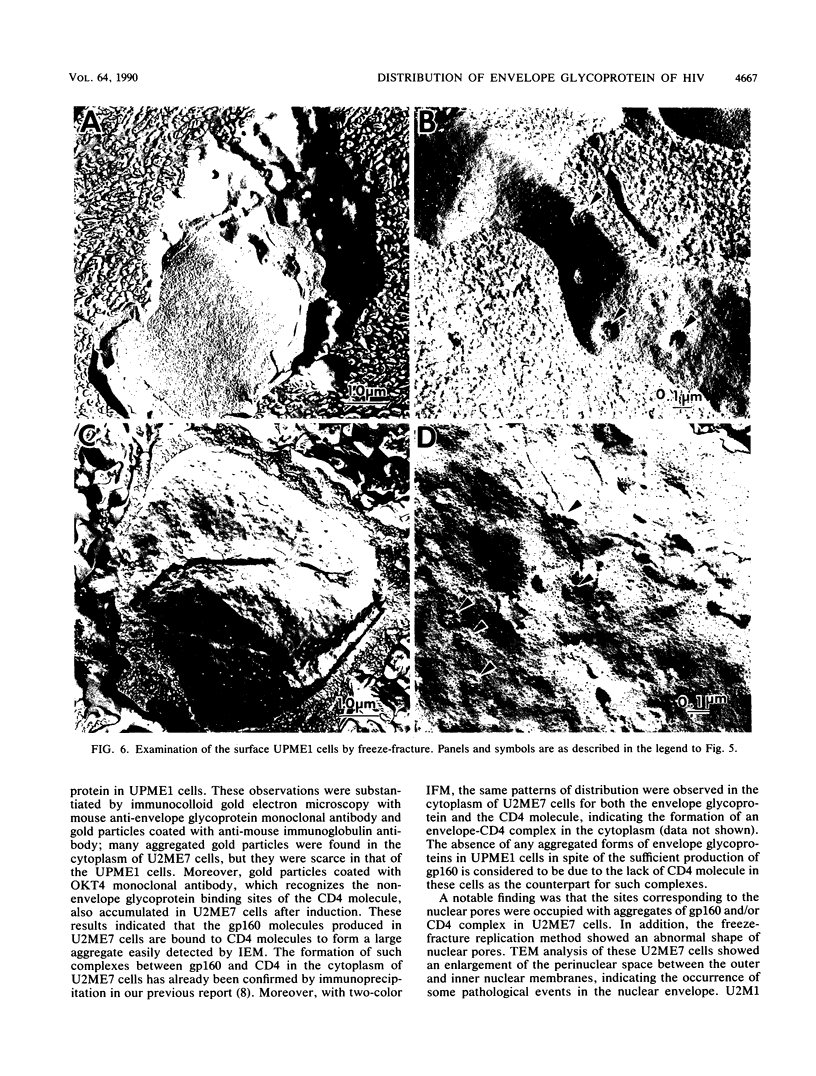
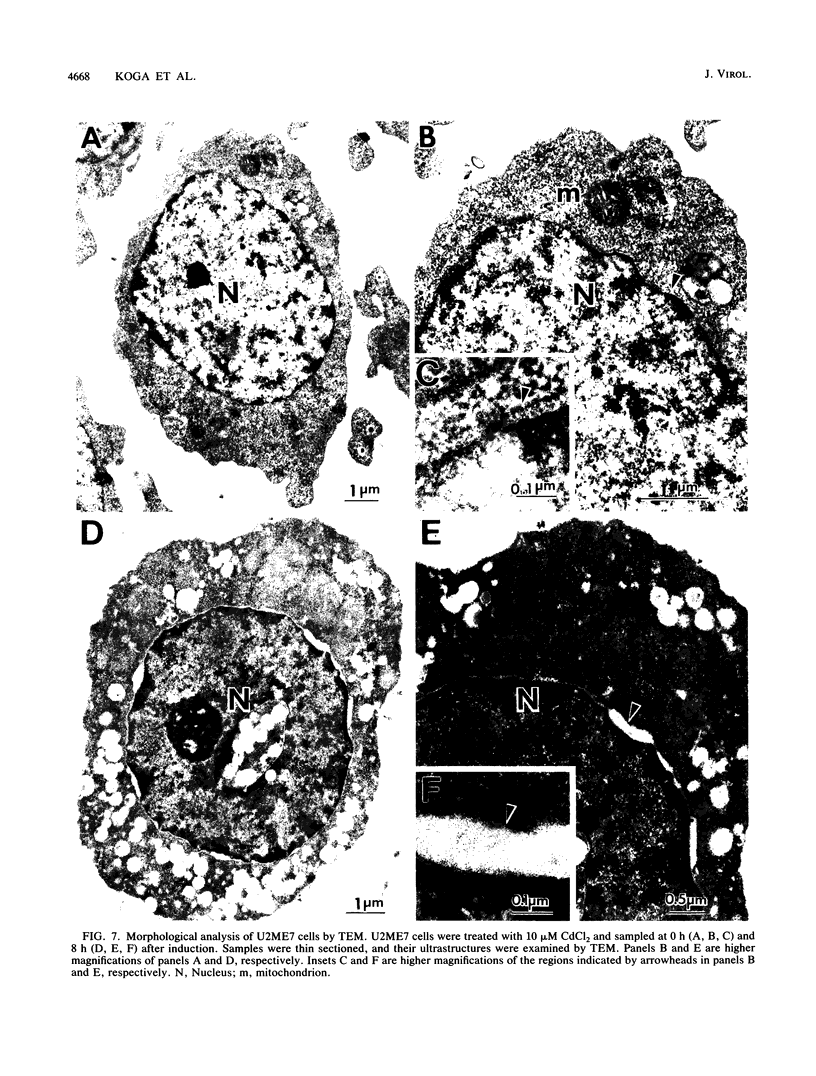
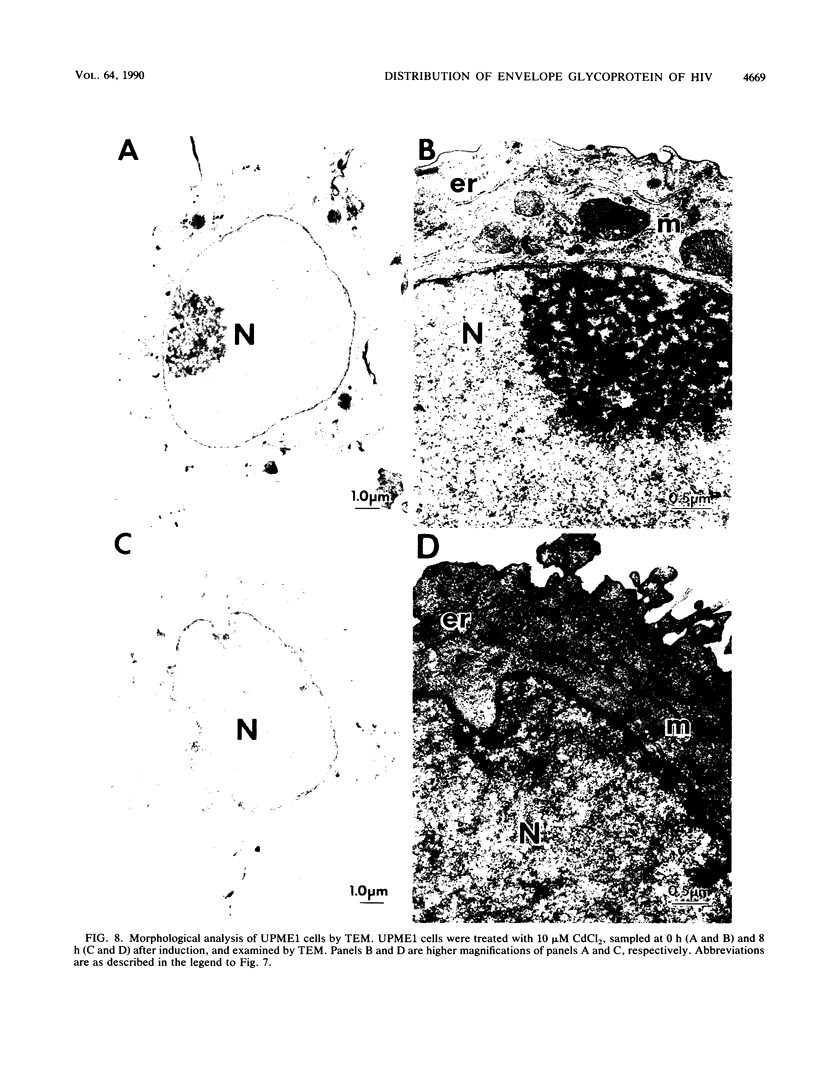
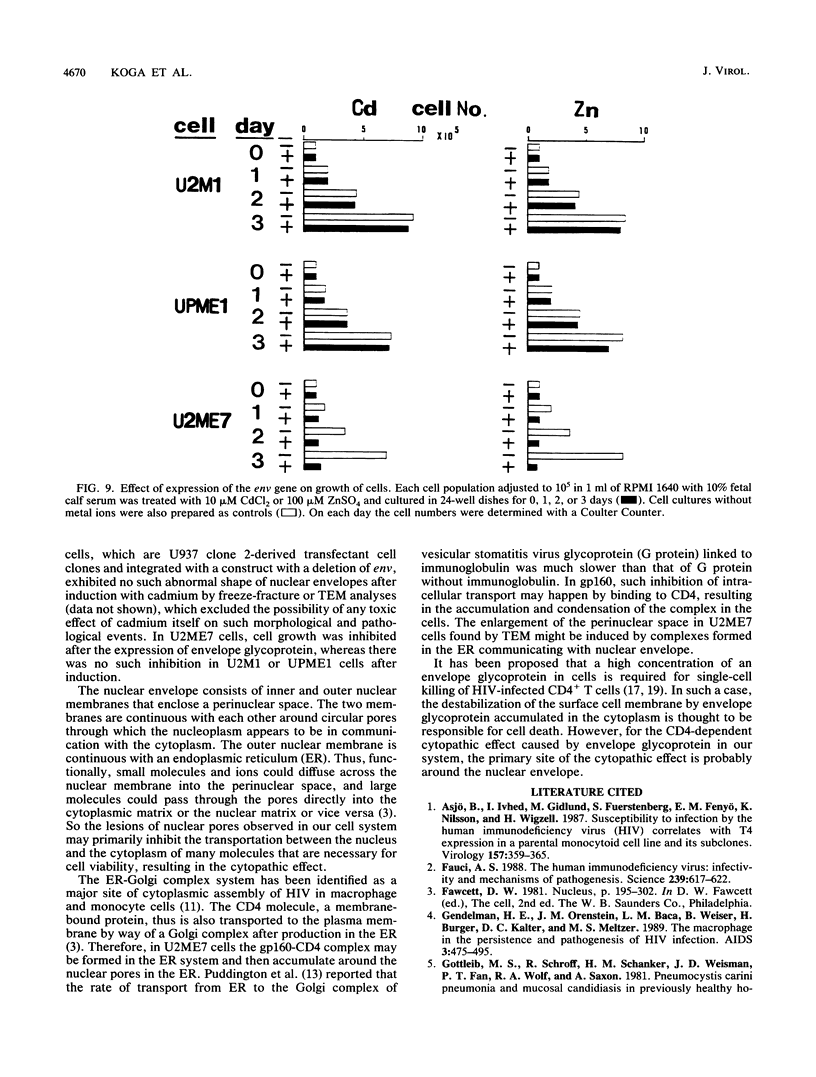
Abstract
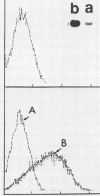
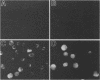
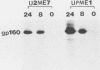
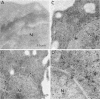
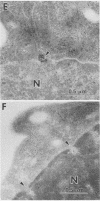
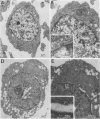
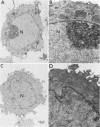
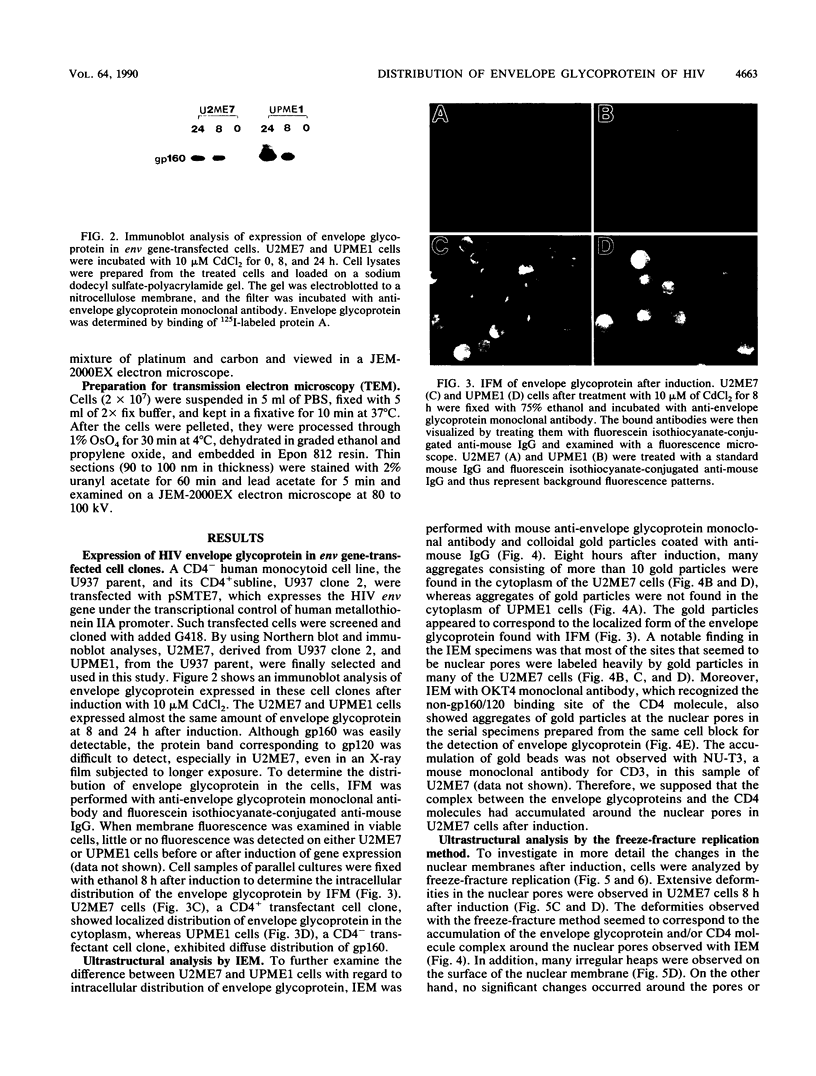
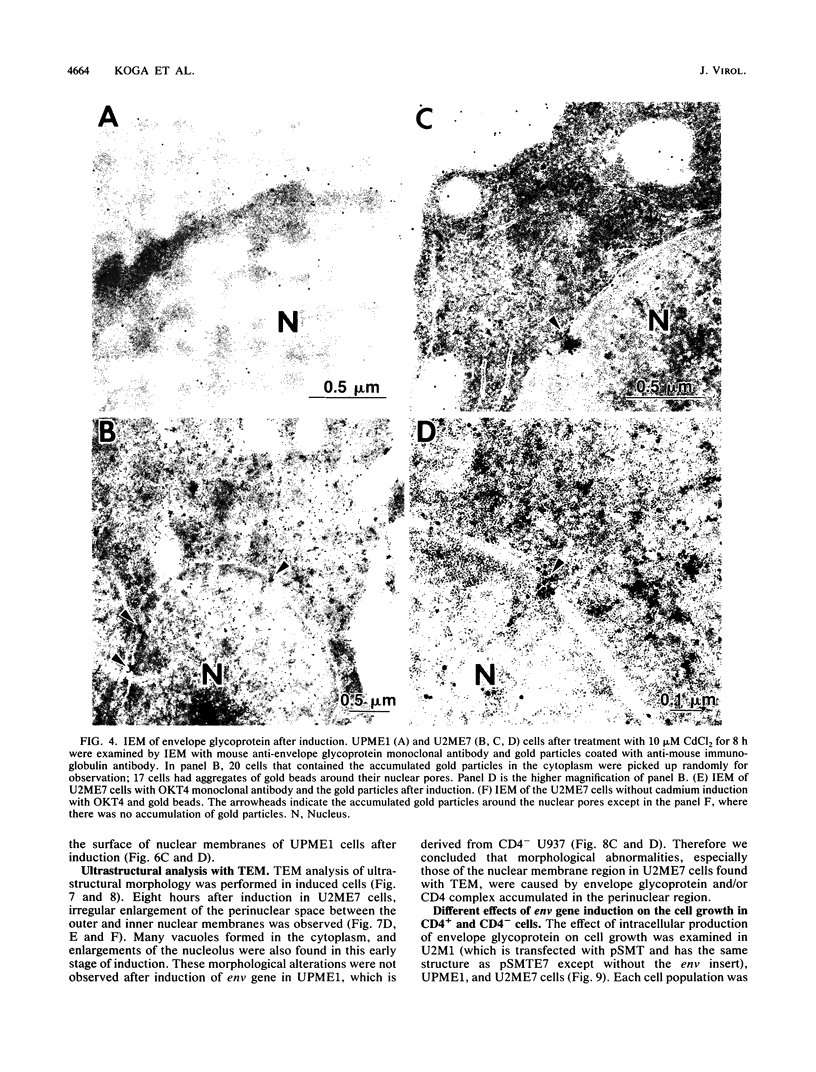
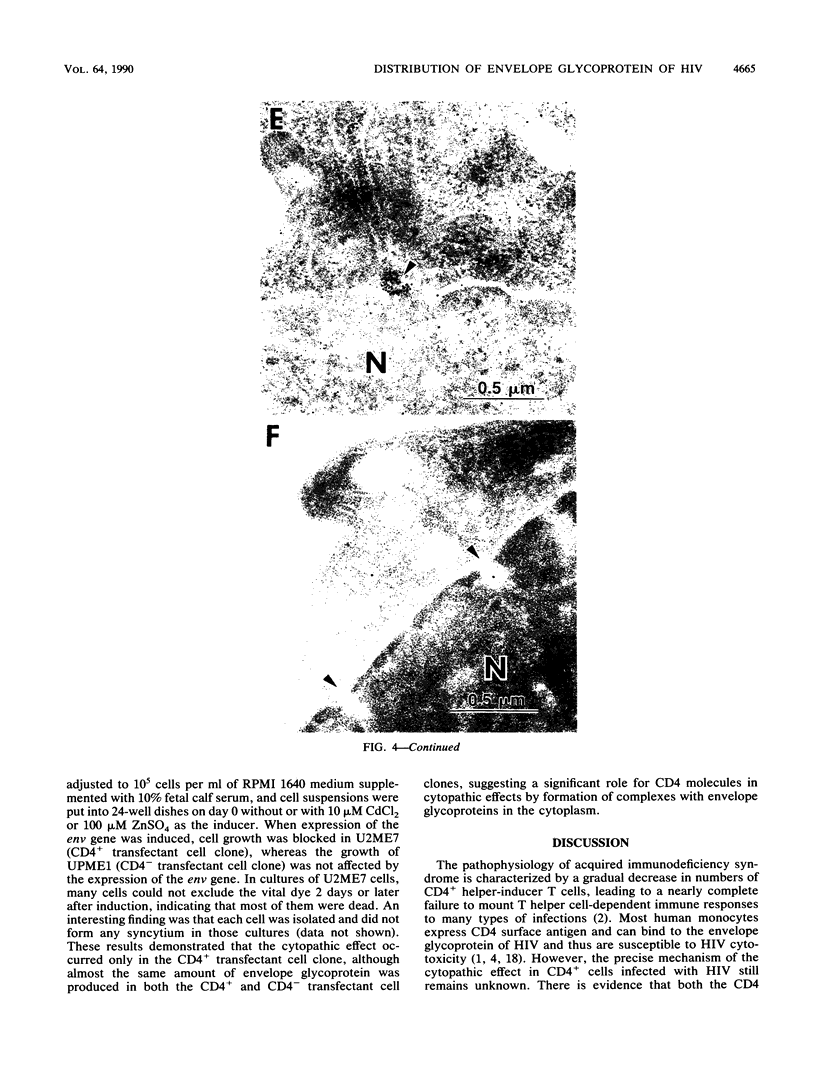
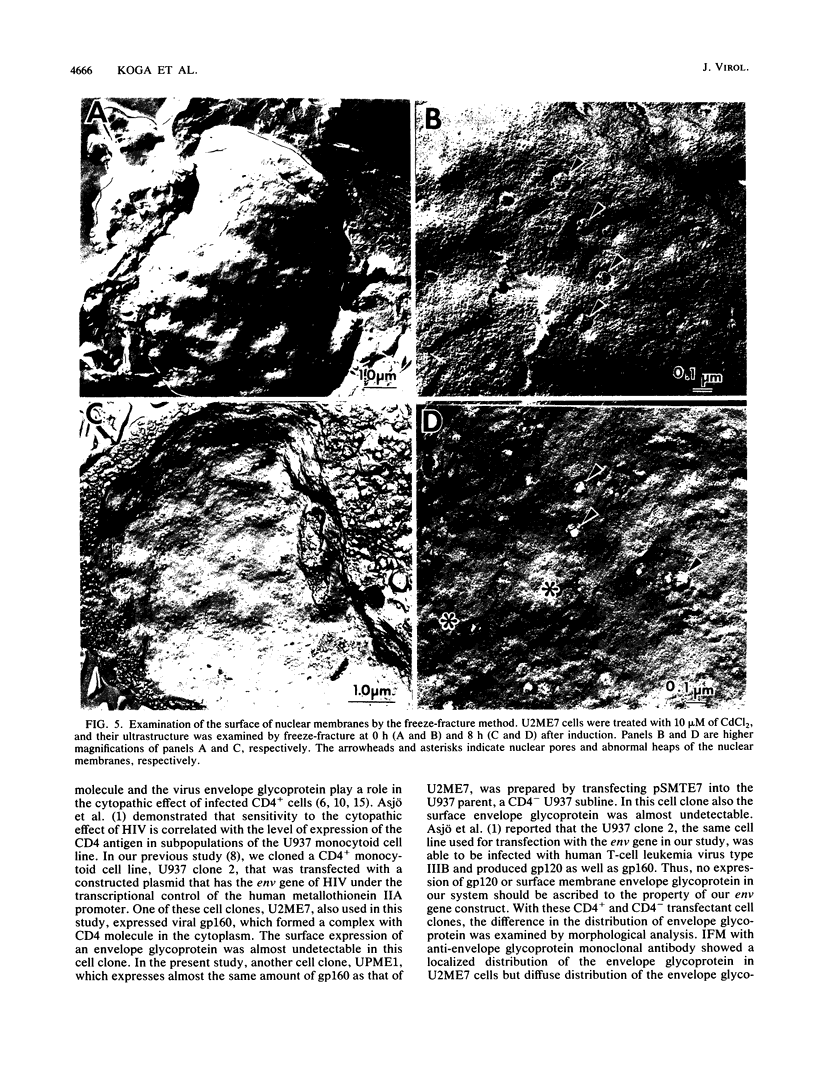
Human CD4+ and CD4- monocytoid cell lines were transfected with a constructed plasmid that has the envelope gene of human immunodeficiency virus under the transcriptional control of human metallothionein IIA promoter; the transfected cells were then cloned. These CD4+ and CD4- transfectant cell clones, both of which expressed almost the same amount of gp160 after induction with metal ions, were used for ultrastructural analysis of the distribution of the envelope glycoprotein in the cytoplasm. Immunofluorescence microscopy with an anti-envelope glycoprotein monoclonal antibody showed localized distribution of gp160 in the CD4+ cell clone and diffuse distribution of gp160 in the CD4- cell clone. These observations were substantiated by immunoelectron microscopy, in which the aggregated form of gp160 was observed in the cytoplasm of CD4+ cells but was scarce in that of CD4- cells. A notable finding was that the sites corresponding to the nuclear pores were occupied with aggregates of gp160 in CD4+ cells, exhibiting cytopathic effects. Both freeze-fracture and transmission electron microscopy also showed abnormal morphology around the nuclear pores and perinuclear space. These results support the possibility that such gp160 complexes accumulated around the nuclear pores primarily disturb the transportation of many molecules between the nucleus and the cytoplasm, resulting in a cytopathic effect in the CD4+ cell clone.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asjö B., Ivhed I., Gidlund M., Fuerstenberg S., Fenyö E. M., Nilsson K., Wigzell H. Susceptibility to infection by the human immunodeficiency virus (HIV) correlates with T4 expression in a parental monocytoid cell line and its subclones. Virology. 1987 Apr;157(2):359–365. doi: 10.1016/0042-6822(87)90278-9. [DOI] [PubMed] [Google Scholar]
- De Rossi A., Franchini G., Aldovini A., Del Mistro A., Chieco-Bianchi L., Gallo R. C., Wong-Staal F. Differential response to the cytopathic effects of human T-cell lymphotropic virus type III (HTLV-III) superinfection in T4+ (helper) and T8+ (suppressor) T-cell clones transformed by HTLV-I. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4297–4301. doi: 10.1073/pnas.83.12.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988 Feb 5;239(4840):617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Orenstein J. M., Baca L. M., Weiser B., Burger H., Kalter D. C., Meltzer M. S. The macrophage in the persistence and pathogenesis of HIV infection. AIDS. 1989 Aug;3(8):475–495. doi: 10.1097/00002030-198908000-00001. [DOI] [PubMed] [Google Scholar]
- Gottlieb M. S., Schroff R., Schanker H. M., Weisman J. D., Fan P. T., Wolf R. A., Saxon A. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med. 1981 Dec 10;305(24):1425–1431. doi: 10.1056/NEJM198112103052401. [DOI] [PubMed] [Google Scholar]
- Hoxie J. A., Alpers J. D., Rackowski J. L., Huebner K., Haggarty B. S., Cedarbaum A. J., Reed J. C. Alterations in T4 (CD4) protein and mRNA synthesis in cells infected with HIV. Science. 1986 Nov 28;234(4780):1123–1127. doi: 10.1126/science.3095925. [DOI] [PubMed] [Google Scholar]
- Karin M., Richards R. I. Human metallothionein genes--primary structure of the metallothionein-II gene and a related processed gene. Nature. 1982 Oct 28;299(5886):797–802. doi: 10.1038/299797a0. [DOI] [PubMed] [Google Scholar]
- Kawamura I., Koga Y., Oh-Hori N., Onodera K., Kimura G., Nomoto K. Depletion of the surface CD4 molecule by the envelope protein of human immunodeficiency virus expressed in a human CD4+ monocytoid cell line. J Virol. 1989 Sep;63(9):3748–3754. doi: 10.1128/jvi.63.9.3748-3754.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masur H., Michelis M. A., Greene J. B., Onorato I., Stouwe R. A., Holzman R. S., Wormser G., Brettman L., Lange M., Murray H. W. An outbreak of community-acquired Pneumocystis carinii pneumonia: initial manifestation of cellular immune dysfunction. N Engl J Med. 1981 Dec 10;305(24):1431–1438. doi: 10.1056/NEJM198112103052402. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Kennedy M. S., Sligh J. M., Cort S. P., Mawle A., Nicholson J. K. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science. 1986 Jan 24;231(4736):382–385. doi: 10.1126/science.3001934. [DOI] [PubMed] [Google Scholar]
- Orenstein J. M., Meltzer M. S., Phipps T., Gendelman H. E. Cytoplasmic assembly and accumulation of human immunodeficiency virus types 1 and 2 in recombinant human colony-stimulating factor-1-treated human monocytes: an ultrastructural study. J Virol. 1988 Aug;62(8):2578–2586. doi: 10.1128/jvi.62.8.2578-2586.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puddington L., Machamer C. E., Rose J. K. Cytoplasmic domains of cellular and viral integral membrane proteins substitute for the cytoplasmic domain of the vesicular stomatitis virus glycoprotein in transport to the plasma membrane. J Cell Biol. 1986 Jun;102(6):2147–2157. doi: 10.1083/jcb.102.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rácz P., Tenner-Rácz K., Kahl C., Feller A. C., Kern P., Dietrich M. Spectrum of morphologic changes of lymph nodes from patients with AIDS or AIDS-related complexes. Prog Allergy. 1986;37:81–181. doi: 10.1159/000318442. [DOI] [PubMed] [Google Scholar]
- Somasundaran M., Robinson H. L. A major mechanism of human immunodeficiency virus-induced cell killing does not involve cell fusion. J Virol. 1987 Oct;61(10):3114–3119. doi: 10.1128/jvi.61.10.3114-3119.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M., Meier C., Mann A. M., Chapman N., Wasiak A. Envelope glycoprotein of HIV induces interference and cytolysis resistance in CD4+ cells: mechanism for persistence in AIDS. Cell. 1988 May 6;53(3):483–496. doi: 10.1016/0092-8674(88)90168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S. J., Fujimoto J., Levy R. Human T lymphocytes and monocytes bear the same Leu-3(T4) antigen. J Immunol. 1986 May 15;136(10):3773–3778. [PubMed] [Google Scholar]
- Terwilliger E. F., Cohen E. A., Lu Y. C., Sodroski J. G., Haseltine W. A. Functional role of human immunodeficiency virus type 1 vpu. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5163–5167. doi: 10.1073/pnas.86.13.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]