Abstract
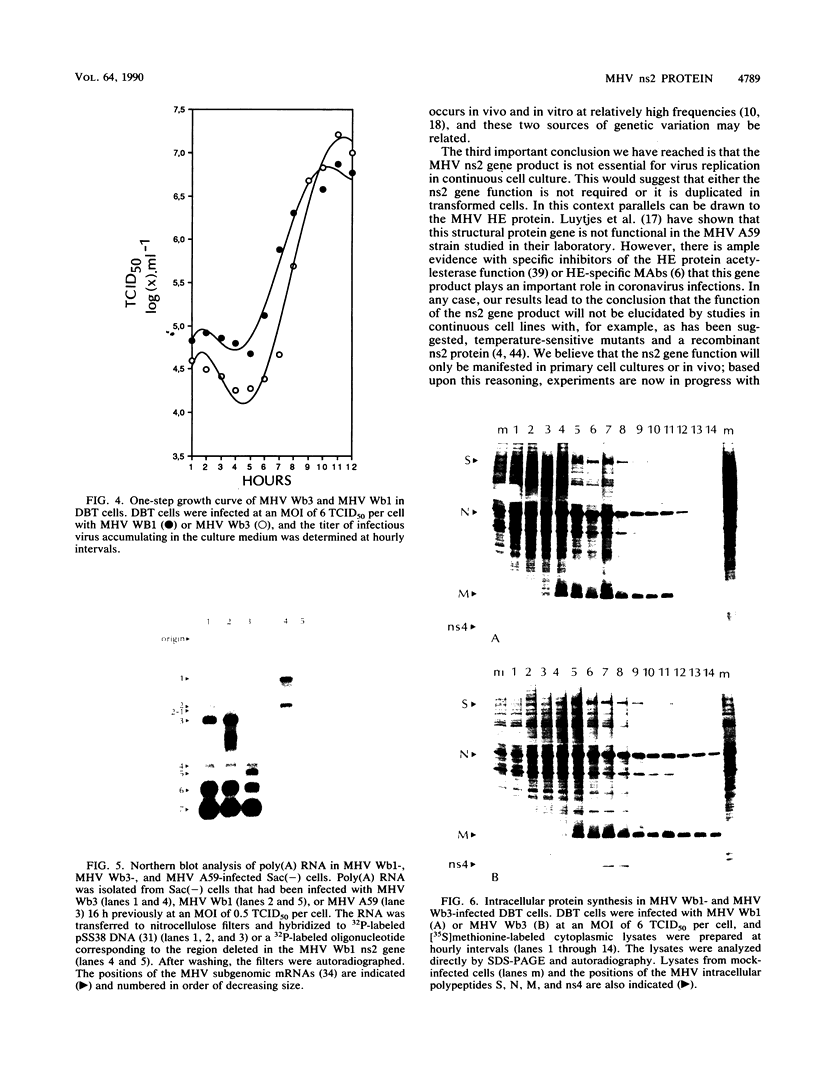
Two isolates of the murine hepatitis virus (MHV) strain JHM, which differed in their ability to express the nonstructural gene product ns2, were characterized. The MHV Wb3 isolate encodes a 30,000-molecular-weight ns2 protein that can be readily detected in infected cells by using a specific monoclonal antibody, MAb 2A. The MHV Wb1 isolate is a deletion mutant that lacks a functional ns2 gene and the transcriptional signals required for the synthesis of an ns2 mRNA. However, there are no obviously significant differences in the growth of the MHV Wb1 and MHV Wb3 isolates in continuous cell lines or in the synthesis of viral mRNAs or proteins in infected cells. These results demonstrate that the ns2 gene product is not essential for MHV replication in transformed murine cells and suggests that the function of the ns2 gene may only be manifest in vivo.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baric R. S., Stohlman S. A., Lai M. M. Characterization of replicative intermediate RNA of mouse hepatitis virus: presence of leader RNA sequences on nascent chains. J Virol. 1983 Dec;48(3):633–640. doi: 10.1128/jvi.48.3.633-640.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard H. U., Helinski D. R. Use of the lambda phage promoter PL to promote gene expression in hybrid plasmid cloning vehicles. Methods Enzymol. 1979;68:482–492. doi: 10.1016/0076-6879(79)68037-0. [DOI] [PubMed] [Google Scholar]
- Boursnell M. E., Binns M. M., Foulds I. J., Brown T. D. Sequences of the nucleocapsid genes from two strains of avian infectious bronchitis virus. J Gen Virol. 1985 Mar;66(Pt 3):573–580. doi: 10.1099/0022-1317-66-3-573. [DOI] [PubMed] [Google Scholar]
- Bredenbeek P. J., Noten A. F., Horzinek M. C., Spaan W. J. Identification and stability of a 30-kDa nonstructural protein encoded by mRNA 2 of mouse hepatitis virus in infected cells. Virology. 1990 Mar;175(1):303–306. doi: 10.1016/0042-6822(90)90212-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D., Brian D. A., Enjuanes L., Holmes K. V., Lai M. M., Laude H., Siddell S. G., Spaan W., Taguchi F., Talbot P. J. Recommendations of the Coronavirus Study Group for the nomenclature of the structural proteins, mRNAs, and genes of coronaviruses. Virology. 1990 May;176(1):306–307. doi: 10.1016/0042-6822(90)90259-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowl R., Seamans C., Lomedico P., McAndrew S. Versatile expression vectors for high-level synthesis of cloned gene products in Escherichia coli. Gene. 1985;38(1-3):31–38. doi: 10.1016/0378-1119(85)90200-8. [DOI] [PubMed] [Google Scholar]
- Deregt D., Babiuk L. A. Monoclonal antibodies to bovine coronavirus: characteristics and topographical mapping of neutralizing epitopes on the E2 and E3 glycoproteins. Virology. 1987 Dec;161(2):410–420. doi: 10.1016/0042-6822(87)90134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner D., Raabe T., Siddell S. G. Identification of the coronavirus MHV-JHM mRNA 4 product. J Gen Virol. 1988 May;69(Pt 5):1041–1050. doi: 10.1099/0022-1317-69-5-1041. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Keck J. G., Matsushima G. K., Makino S., Fleming J. O., Vannier D. M., Stohlman S. A., Lai M. M. In vivo RNA-RNA recombination of coronavirus in mouse brain. J Virol. 1988 May;62(5):1810–1813. doi: 10.1128/jvi.62.5.1810-1813.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumanishi T. Brain tumors induced with Rous sarcoma virus, Schmidt-Ruppin strain. I. Induction of brain tumors in adult mice with Rous chicken sarcoma cells. Jpn J Exp Med. 1967 Oct;37(5):461–474. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai M. M. Coronavirus leader-RNA-primed transcription: an alternative mechanism to RNA splicing. Bioessays. 1986 Dec;5(6):257–260. doi: 10.1002/bies.950050606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Patton C. D., Baric R. S., Stohlman S. A. Presence of leader sequences in the mRNA of mouse hepatitis virus. J Virol. 1983 Jun;46(3):1027–1033. doi: 10.1128/jvi.46.3.1027-1033.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz J. L., Perlman S., Weinstock G., DeVries J. R., Budzilowicz C., Weissemann J. M., Weiss S. R. Detection of a murine coronavirus nonstructural protein encoded in a downstream open reading frame. Virology. 1988 May;164(1):156–164. doi: 10.1016/0042-6822(88)90631-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz J. L., Weiss S. R., Paavola E., Bond C. W. Cell-free translation of murine coronavirus RNA. J Virol. 1982 Sep;43(3):905–913. doi: 10.1128/jvi.43.3.905-913.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luytjes W., Bredenbeek P. J., Noten A. F., Horzinek M. C., Spaan W. J. Sequence of mouse hepatitis virus A59 mRNA 2: indications for RNA recombination between coronaviruses and influenza C virus. Virology. 1988 Oct;166(2):415–422. doi: 10.1016/0042-6822(88)90512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S., Keck J. G., Stohlman S. A., Lai M. M. High-frequency RNA recombination of murine coronaviruses. J Virol. 1986 Mar;57(3):729–737. doi: 10.1128/jvi.57.3.729-737.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris V. L., Tieszer C., Mackinnon J., Percy D. Characterization of coronavirus JHM variants isolated from Wistar Furth rats with a viral-induced demyelinating disease. Virology. 1989 Mar;169(1):127–136. doi: 10.1016/0042-6822(89)90048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myint S., Harmsen D., Raabe T., Siddell S. G. Characterization of a nucleic acid probe for the diagnosis of human coronavirus 229E infections. J Med Virol. 1990 Jun;31(2):165–172. doi: 10.1002/jmv.1890310216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachuk C. J., Bredenbeek P. J., Zoltick P. W., Spaan W. J., Weiss S. R. Molecular cloning of the gene encoding the putative polymerase of mouse hepatitis coronavirus, strain A59. Virology. 1989 Jul;171(1):141–148. doi: 10.1016/0042-6822(89)90520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker S. E., Gallagher T. M., Buchmeier M. J. Sequence analysis reveals extensive polymorphism and evidence of deletions within the E2 glycoprotein gene of several strains of murine hepatitis virus. Virology. 1989 Dec;173(2):664–673. doi: 10.1016/0042-6822(89)90579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson A. C., Willcocks M. M., Routledge E. G., Morgan L. A., Toms G. L. A neutralizing monoclonal antibody to respiratory syncytial virus which binds to both F1 and F2 components of the fusion protein. J Gen Virol. 1986 Jul;67(Pt 7):1479–1483. doi: 10.1099/0022-1317-67-7-1479. [DOI] [PubMed] [Google Scholar]
- Sawicki S. G., Sawicki D. L. Coronavirus transcription: subgenomic mouse hepatitis virus replicative intermediates function in RNA synthesis. J Virol. 1990 Mar;64(3):1050–1056. doi: 10.1128/jvi.64.3.1050-1056.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh C. K., Lee H. J., Yokomori K., La Monica N., Makino S., Lai M. M. Identification of a new transcriptional initiation site and the corresponding functional gene 2b in the murine coronavirus RNA genome. J Virol. 1989 Sep;63(9):3729–3736. doi: 10.1128/jvi.63.9.3729-3736.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddell S. G. Coronavirus JHM: tryptic peptide fingerprinting of virion proteins and intracellular polypeptides. J Gen Virol. 1982 Oct;62(Pt 2):259–269. doi: 10.1099/0022-1317-62-2-259. [DOI] [PubMed] [Google Scholar]
- Siddell S. G., Wege H., Barthel A., ter Meulen V. Coronavirus JHM: cell-free synthesis of structural protein p60. J Virol. 1980 Jan;33(1):10–17. doi: 10.1128/jvi.33.1.10-17.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddell S. Coronavirus JHM: coding assignments of subgenomic mRNAs. J Gen Virol. 1983 Jan;64(Pt 1):113–125. doi: 10.1099/0022-1317-64-1-113. [DOI] [PubMed] [Google Scholar]
- Siddell S., Wege H., Barthel A., ter Meulen V. Coronavirus JHM: intracellular protein synthesis. J Gen Virol. 1981 Mar;53(Pt 1):145–155. doi: 10.1099/0022-1317-53-1-145. [DOI] [PubMed] [Google Scholar]
- Skinner M. A., Siddell S. G. Coronavirus JHM: nucleotide sequence of the mRNA that encodes nucleocapsid protein. Nucleic Acids Res. 1983 Aug 11;11(15):5045–5054. doi: 10.1093/nar/11.15.5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soe L. H., Shieh C. K., Baker S. C., Chang M. F., Lai M. M. Sequence and translation of the murine coronavirus 5'-end genomic RNA reveals the N-terminal structure of the putative RNA polymerase. J Virol. 1987 Dec;61(12):3968–3976. doi: 10.1128/jvi.61.12.3968-3976.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaan W., Cavanagh D., Horzinek M. C. Coronaviruses: structure and genome expression. J Gen Virol. 1988 Dec;69(Pt 12):2939–2952. doi: 10.1099/0022-1317-69-12-2939. [DOI] [PubMed] [Google Scholar]
- Spaan W., Delius H., Skinner M., Armstrong J., Rottier P., Smeekens S., van der Zeijst B. A., Siddell S. G. Coronavirus mRNA synthesis involves fusion of non-contiguous sequences. EMBO J. 1983;2(10):1839–1844. doi: 10.1002/j.1460-2075.1983.tb01667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley K. K., Luzio J. P. Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J. 1984 Jun;3(6):1429–1434. doi: 10.1002/j.1460-2075.1984.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi F., Massa P. T., ter Meulen V. Characterization of a variant virus isolated from neural cell culture after infection of mouse coronavirus JHMV. Virology. 1986 Nov;155(1):267–270. doi: 10.1016/0042-6822(86)90187-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi F., Siddell S. G., Wege H., ter Meulen V. Characterization of a variant virus selected in rat brains after infection by coronavirus mouse hepatitis virus JHM. J Virol. 1985 May;54(2):429–435. doi: 10.1128/jvi.54.2.429-435.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasak R., Luytjes W., Leider J., Spaan W., Palese P. The E3 protein of bovine coronavirus is a receptor-destroying enzyme with acetylesterase activity. J Virol. 1988 Dec;62(12):4686–4690. doi: 10.1128/jvi.62.12.4686-4690.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wege H., Siddell S., ter Meulen V. The biology and pathogenesis of coronaviruses. Curr Top Microbiol Immunol. 1982;99:165–200. doi: 10.1007/978-3-642-68528-6_5. [DOI] [PubMed] [Google Scholar]
- Weiland E., Mussgay M., Weiland F. Nonproducer malignant tumor cells with rescuable sarcoma virus genome isolated from a recurrent Moloney sarcoma. J Exp Med. 1978 Aug 1;148(2):408–423. doi: 10.1084/jem.148.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoltick P. W., Leibowitz J. L., Oleszak E. L., Weiss S. R. Mouse hepatitis virus ORF 2a is expressed in the cytosol of infected mouse fibroblasts. Virology. 1990 Feb;174(2):605–607. doi: 10.1016/0042-6822(90)90114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]