Abstract
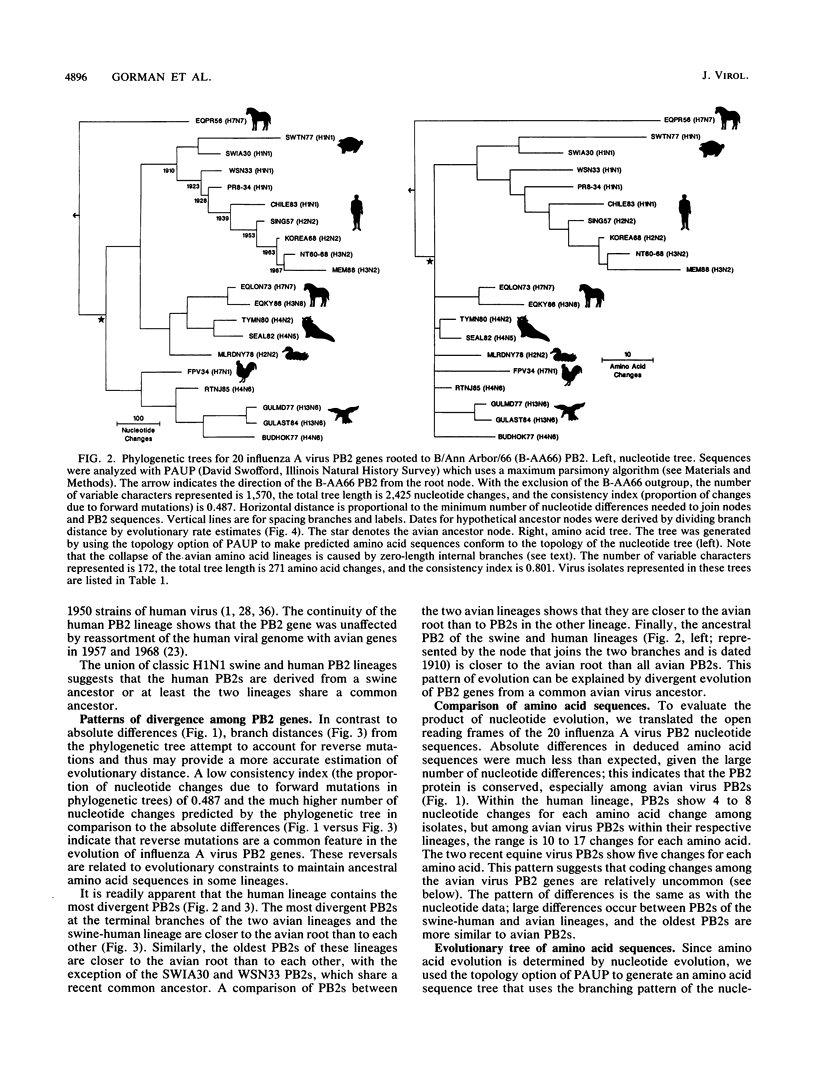
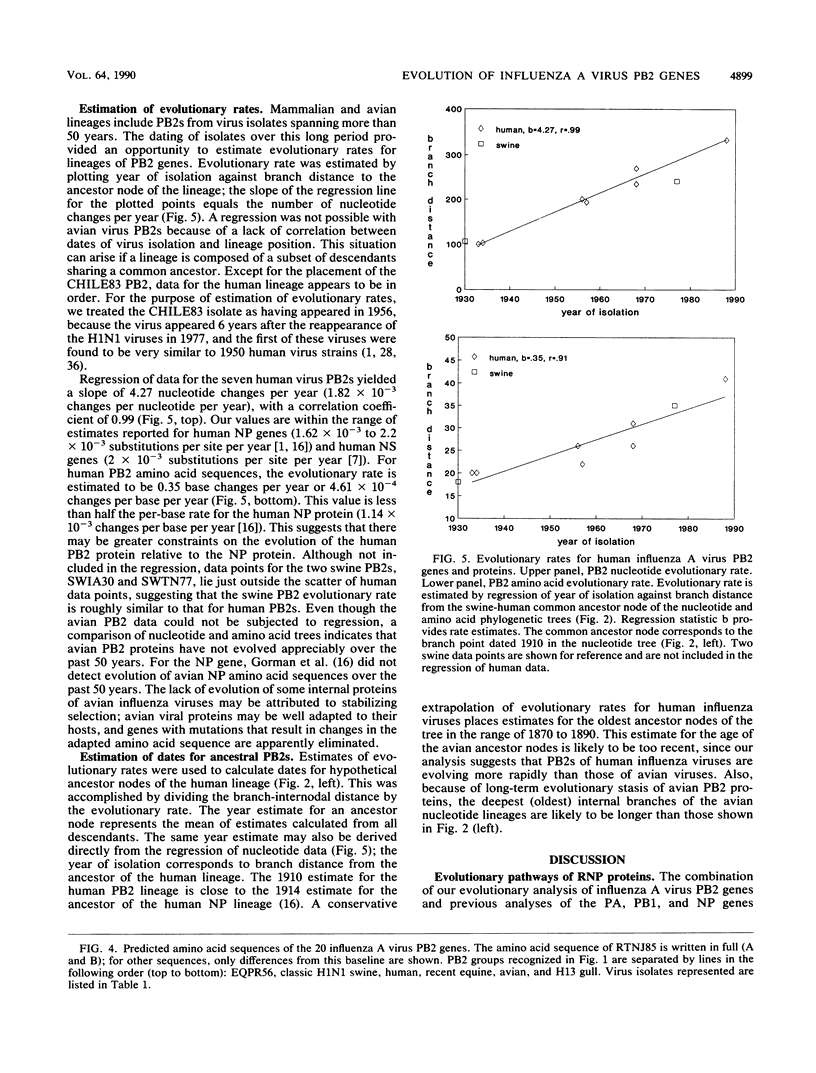
Phylogenetic analysis of 20 influenza A virus PB2 genes showed that PB2 genes have evolved into the following four major lineages: (i) equine/Prague/56 (EQPR56); (ii and iii) two distinct avian PB2 lineages, one containing FPV/34 and H13 gull virus strains and the other containing North American avian and recent equine strains; and (iv) human virus strains joined with classic swine virus strains (i.e., H1N1 swine virus strains related to swine/Iowa/15/30). The human virus lineage showed the greatest divergence from its root relative to other lineages. The estimated nucleotide evolutionary rate for the human PB2 lineage was 1.82 x 10(-3) changes per nucleotide per year, which is within the range of published estimates for NP and NS genes of human influenza A viruses. At the amino acid level, PB2s of human viruses have accumulated 34 amino acid changes over the past 55 years. In contrast, the avian PB2 lineages showed much less evolution, e.g., recent avian PB2s showed as few as three amino acid changes relative to the avian root. The completion of evolutionary analyses of the PB1, PB2, PA and NP genes of the ribonucleoprotein (RNP) complex permits comparison of evolutionary pathways. Different patterns of evolution among the RNP genes indicate that the genes of the complex are not coevolving as a unit. Evolution of the PB1 and PB2 genes is less correlated with host-specific factors, and their proteins appear to be evolving more slowly than NP and PA. This suggests that protein functional constraints are limiting the evolutionary divergence of PB1 and PB2 genes. The parallel host-specific evolutionary pathways of the NP and PA genes suggest that these proteins are coevolving in response to host-specific factors. PB2s of human influenza A viruses share a common ancestor with classic swine virus PB2s, and the pattern of evolution suggests that the ancestor was an avian virus PB2. This same pattern of evolution appears in the other genes of the RNP complex. Antigenic studies of HA and NA proteins and sequence comparisons of NS and M genes also suggest a close ancestry for these genes in human and classic swine viruses. From our review of the evolutionary patterns of influenza A virus genes, we propose the following hypothesis: the common ancestor to current strains of human and classic swine influenza viruses predated the 1918 human pandemic virus and was recently derived from the avian host reservoir.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmüller A., Fitch W. M., Scholtissek C. Biological and genetic evolution of the nucleoprotein gene of human influenza A viruses. J Gen Virol. 1989 Aug;70(Pt 8):2111–2119. doi: 10.1099/0022-1317-70-8-2111. [DOI] [PubMed] [Google Scholar]
- Bean W. J. Correlation of influenza A virus nucleoprotein genes with host species. Virology. 1984 Mar;133(2):438–442. doi: 10.1016/0042-6822(84)90410-0. [DOI] [PubMed] [Google Scholar]
- Bean W. J., Jr, Sriram G., Webster R. G. Electrophoretic analysis of iodine-labeled influenza virus RNA segments. Anal Biochem. 1980 Feb;102(1):228–232. doi: 10.1016/0003-2697(80)90343-7. [DOI] [PubMed] [Google Scholar]
- Bean W. J., Kawaoka Y., Wood J. M., Pearson J. E., Webster R. G. Characterization of virulent and avirulent A/chicken/Pennsylvania/83 influenza A viruses: potential role of defective interfering RNAs in nature. J Virol. 1985 Apr;54(1):151–160. doi: 10.1128/jvi.54.1.151-160.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaas D., Patzelt E., Kuechler E. Cap-recognizing protein of influenza virus. Virology. 1982 Jan 15;116(1):339–348. doi: 10.1016/0042-6822(82)90425-1. [DOI] [PubMed] [Google Scholar]
- Braam J., Ulmanen I., Krug R. M. Molecular model of a eucaryotic transcription complex: functions and movements of influenza P proteins during capped RNA-primed transcription. Cell. 1983 Sep;34(2):609–618. doi: 10.1016/0092-8674(83)90393-8. [DOI] [PubMed] [Google Scholar]
- Buonagurio D. A., Nakada S., Parvin J. D., Krystal M., Palese P., Fitch W. M. Evolution of human influenza A viruses over 50 years: rapid, uniform rate of change in NS gene. Science. 1986 May 23;232(4753):980–982. doi: 10.1126/science.2939560. [DOI] [PubMed] [Google Scholar]
- Chambers T. M., Yamnikova S., Kawaoka Y., Lvov D. K., Webster R. G. Antigenic and molecular characterization of subtype H13 hemagglutinin of influenza virus. Virology. 1989 Sep;172(1):180–188. doi: 10.1016/0042-6822(89)90119-0. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- DAVENPORT F. M., HENNESSY A. V., DRESCHER J., MULDER J., FRANCIS T., Jr FURTHER OBSERVATIONS ON THE RELEVANCE OF SEROLOGIC RECAPITULATIONS OF HUMAN INFECTION WITH INFLUENZA VIRUSES. J Exp Med. 1964 Dec 1;120:1087–1097. doi: 10.1084/jem.120.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVENPORT F. M., HENNESSY A. V., FRANCIS T., Jr Epidemiologic and immunologic significance of age distribution of antibody to antigenic variants of influenza virus. J Exp Med. 1953 Dec;98(6):641–656. doi: 10.1084/jem.98.6.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacso C. C., Couch R. B., Six H. R., Young J. F., Quarles J. M., Kasel J. A. Sporadic occurrence of zoonotic swine influenza virus infections. J Clin Microbiol. 1984 Oct;20(4):833–835. doi: 10.1128/jcm.20.4.833-835.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBorde D. C., Donabedian A. M., Herlocher M. L., Naeve C. W., Maassab H. F. Sequence comparison of wild-type and cold-adapted B/Ann Arbor/1/66 influenza virus genes. Virology. 1988 Apr;163(2):429–443. doi: 10.1016/0042-6822(88)90284-x. [DOI] [PubMed] [Google Scholar]
- Fields S., Winter G. Nucleotide sequences of influenza virus segments 1 and 3 reveal mosaic structure of a small viral RNA segment. Cell. 1982 Feb;28(2):303–313. doi: 10.1016/0092-8674(82)90348-8. [DOI] [PubMed] [Google Scholar]
- Gammelin M., Mandler J., Scholtissek C. Two subtypes of nucleoproteins (NP) of influenza A viruses. Virology. 1989 May;170(1):71–80. doi: 10.1016/0042-6822(89)90353-x. [DOI] [PubMed] [Google Scholar]
- Gorman O. T., Bean W. J., Kawaoka Y., Webster R. G. Evolution of the nucleoprotein gene of influenza A virus. J Virol. 1990 Apr;64(4):1487–1497. doi: 10.1128/jvi.64.4.1487-1497.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENNESSY A. V., DAVENPORT F. M. Epidemiologic implications of the distribution by age of antibody response to experimental influenza virus vaccines. J Immunol. 1958 Feb;80(2):114–121. [PubMed] [Google Scholar]
- HENNESSY A. V., DAVENPORT F. M., FRANCIS T., Jr Studies of antibodies to strains of influenza virus in persons of different ages in sera collected in a postepidemic period. J Immunol. 1955 Nov;75(5):401–409. [PubMed] [Google Scholar]
- Hinshaw V. S., Bean W. J., Jr, Webster R. G., Easterday B. C. The prevalence of influenza viruses in swine and the antigenic and genetic relatedness of influenza viruses from man and swine. Virology. 1978 Jan;84(1):51–62. doi: 10.1016/0042-6822(78)90217-9. [DOI] [PubMed] [Google Scholar]
- Jones K. L., Huddleston J. A., Brownlee G. G. The sequence of RNA segment 1 of influenza virus A/NT/60/68 and its comparison with the corresponding segment of strains A/PR/8/34 and A/WSN/33. Nucleic Acids Res. 1983 Mar 11;11(5):1555–1566. doi: 10.1093/nar/11.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptein J. S., Nayak D. P. Complete nucleotide sequence of the polymerase 3 gene of human influenza virus A/WSN/33. J Virol. 1982 Apr;42(1):55–63. doi: 10.1128/jvi.42.1.55-63.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaoka Y., Krauss S., Webster R. G. Avian-to-human transmission of the PB1 gene of influenza A viruses in the 1957 and 1968 pandemics. J Virol. 1989 Nov;63(11):4603–4608. doi: 10.1128/jvi.63.11.4603-4608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida H., Shortridge K. F., Webster R. G. Origin of the hemagglutinin gene of H3N2 influenza viruses from pigs in China. Virology. 1988 Jan;162(1):160–166. doi: 10.1016/0042-6822(88)90405-9. [DOI] [PubMed] [Google Scholar]
- Krystal M., Elliott R. M., Benz E. W., Jr, Young J. F., Palese P. Evolution of influenza A and B viruses: conservation of structural features in the hemagglutinin genes. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4800–4804. doi: 10.1073/pnas.79.15.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULDER J., MASUREL N. Pre-epidemic antibody against 1957 strain of Asiatic influenza in serum of older people living in the Netherlands. Lancet. 1958 Apr 19;1(7025):810–814. doi: 10.1016/s0140-6736(58)91738-0. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Desselberger U., Palese P. Recent human influenza A (H1N1) viruses are closely related genetically to strains isolated in 1950. Nature. 1978 Jul 27;274(5669):334–339. doi: 10.1038/274334a0. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Nobusawa E., Nakajima S. Genetic relatedness between A/Swine/Iowa/15/30(H1N1) and human influenza viruses. Virology. 1984 Nov;139(1):194–198. doi: 10.1016/0042-6822(84)90341-6. [DOI] [PubMed] [Google Scholar]
- Nichol S. T., Penn C. R., Mahy B. W. Evidence for the involvement of influenza A (fowl plague Rostock) virus protein P2 in ApG and mRNA primed in vitro RNA synthesis. J Gen Virol. 1981 Dec;57(Pt 2):407–413. doi: 10.1099/0022-1317-57-2-407. [DOI] [PubMed] [Google Scholar]
- Okazaki K., Kawaoka Y., Webster R. G. Evolutionary pathways of the PA genes of influenza A viruses. Virology. 1989 Oct;172(2):601–608. doi: 10.1016/0042-6822(89)90202-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnurrenberger P. R., Woods G. T., Martin R. J. Serologic evidence of human infection with swine influenza virus. Am Rev Respir Dis. 1970 Sep;102(3):356–361. doi: 10.1164/arrd.1970.102.3.356. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., Bürger H., Bachmann P. A., Hannoun C. Genetic relatedness of hemagglutinins of the H1 subtype of influenza A viruses isolated from swine and birds. Virology. 1983 Sep;129(2):521–523. doi: 10.1016/0042-6822(83)90194-0. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., von Hoyningen V., Rott R. Genetic relatedness between the new 1977 epidemic strains (H1N1) of influenza and human influenza strains isolated between 1947 and 1957 (H1N1). Virology. 1978 Sep;89(2):613–617. doi: 10.1016/0042-6822(78)90203-9. [DOI] [PubMed] [Google Scholar]
- Schreier E., Petzold D. R., Michel S., Dittmann S. Evolution of influenza polymerase: nucleotide sequence of the PB2 gene of A/Chile/1/83 (H1 N1). Arch Virol. 1988;103(3-4):179–187. doi: 10.1007/BF01311091. [DOI] [PubMed] [Google Scholar]
- Treanor J., Kawaoka Y., Miller R., Webster R. G., Murphy B. Nucleotide sequence of the avian influenza A/Mallard/NY/6750/78 virus polymerase genes. Virus Res. 1989 Nov;14(3):257–269. doi: 10.1016/0168-1702(89)90006-3. [DOI] [PubMed] [Google Scholar]
- Ulmanen I., Broni B. A., Krug R. M. Role of two of the influenza virus core P proteins in recognizing cap 1 structures (m7GpppNm) on RNAs and in initiating viral RNA transcription. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7355–7359. doi: 10.1073/pnas.78.12.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. G., Hinshaw V. S., Laver W. G. Selection and analysis of antigenic variants of the neuraminidase of N2 influenza viruses with monoclonal antibodies. Virology. 1982 Feb;117(1):93–104. doi: 10.1016/0042-6822(82)90510-4. [DOI] [PubMed] [Google Scholar]
- Winter G., Fields S., Gait M. J., Brownlee G. G. The use of synthetic oligodeoxynucleotide primers in cloning and sequencing segment of 8 influenza virus (A/PR/8/34). Nucleic Acids Res. 1981 Jan 24;9(2):237–245. doi: 10.1093/nar/9.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]


