Abstract
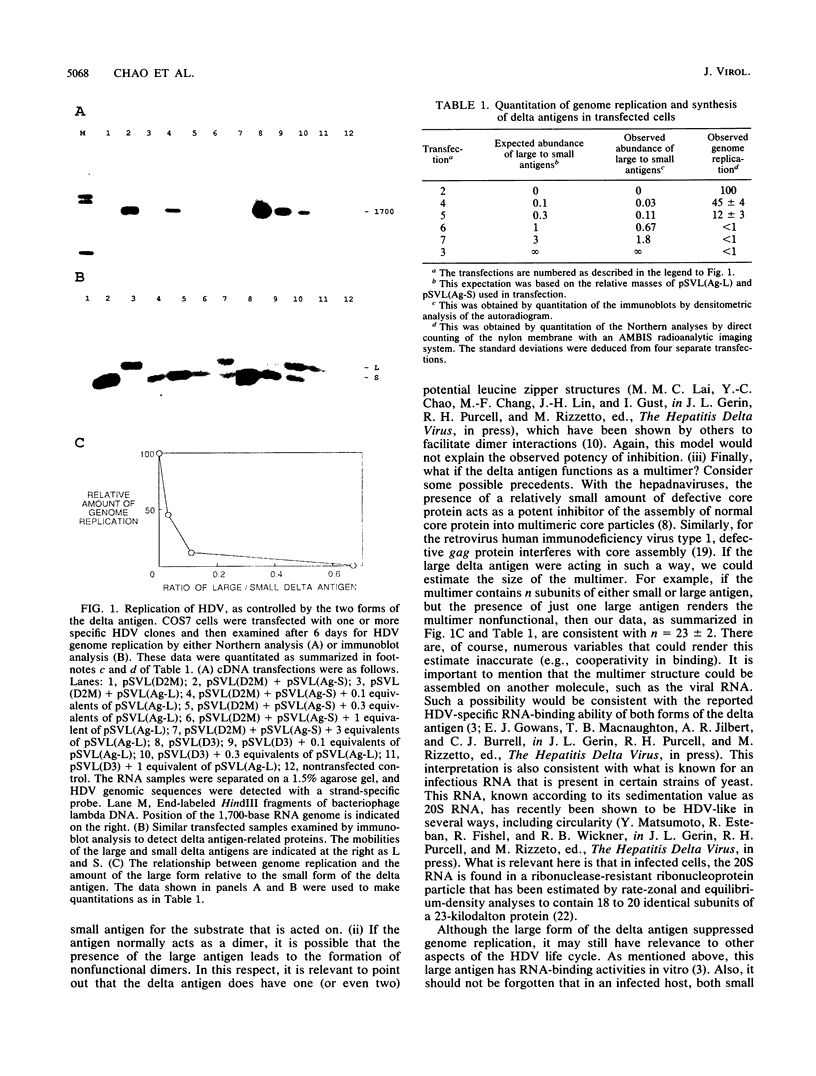
The replication of the RNA genome of hepatitis delta virus is greatly facilitated by the presence of the only known virus-coded protein, the delta antigen. Most, if not all, infections are characterized by the presence of two electrophoretic forms of the delta antigen. These forms correspond to polypeptide lengths of 195 and 214 amino acids which are encoded by genomes with different nucleotide sequences. We used cDNA transfections to investigate the functions of these two forms of the delta antigen. We found that only the small form of delta antigen supported hepatitis delta virus genome replication and that the large form acted as a dominant negative repressor of such replication. This inhibition was potent. For example, the amount of genome replication was reduced eightfold when as little as 10% of the delta antigen was present as the large form. One interpretation of our results is that the delta antigen normally functions as part of a multimeric structure. In addition, our data suggest that synthesis of the large form, either during genome replication in cultured cells or even during infection in animals, may suppress delta replication, possibly leading to a self-limiting infection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergmann K. F., Gerin J. L. Antigens of hepatitis delta virus in the liver and serum of humans and animals. J Infect Dis. 1986 Oct;154(4):702–706. doi: 10.1093/infdis/154.4.702. [DOI] [PubMed] [Google Scholar]
- Bonino F., Heermann K. H., Rizzetto M., Gerlich W. H. Hepatitis delta virus: protein composition of delta antigen and its hepatitis B virus-derived envelope. J Virol. 1986 Jun;58(3):945–950. doi: 10.1128/jvi.58.3.945-950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M. F., Baker S. C., Soe L. H., Kamahora T., Keck J. G., Makino S., Govindarajan S., Lai M. M. Human hepatitis delta antigen is a nuclear phosphoprotein with RNA-binding activity. J Virol. 1988 Jul;62(7):2403–2410. doi: 10.1128/jvi.62.7.2403-2410.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. J., Kalpana G., Goldberg J., Mason W., Werner B., Gerin J., Taylor J. Structure and replication of the genome of the hepatitis delta virus. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8774–8778. doi: 10.1073/pnas.83.22.8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- Glenn J. S., Taylor J. M., White J. M. In vitro-synthesized hepatitis delta virus RNA initiates genome replication in cultured cells. J Virol. 1990 Jun;64(6):3104–3107. doi: 10.1128/jvi.64.6.3104-3107.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz I. Functional inactivation of genes by dominant negative mutations. Nature. 1987 Sep 17;329(6136):219–222. doi: 10.1038/329219a0. [DOI] [PubMed] [Google Scholar]
- Horwich A. L., Furtak K., Pugh J., Summers J. Synthesis of hepadnavirus particles that contain replication-defective duck hepatitis B virus genomes in cultured HuH7 cells. J Virol. 1990 Feb;64(2):642–650. doi: 10.1128/jvi.64.2.642-650.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh S. Y., Chao M., Coates L., Taylor J. Hepatitis delta virus genome replication: a polyadenylated mRNA for delta antigen. J Virol. 1990 Jul;64(7):3192–3198. doi: 10.1128/jvi.64.7.3192-3198.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T., Ziff E. Leucine zippers of fos, jun and GCN4 dictate dimerization specificity and thereby control DNA binding. Nature. 1989 Aug 17;340(6234):568–571. doi: 10.1038/340568a0. [DOI] [PubMed] [Google Scholar]
- Kuo M. Y., Chao M., Taylor J. Initiation of replication of the human hepatitis delta virus genome from cloned DNA: role of delta antigen. J Virol. 1989 May;63(5):1945–1950. doi: 10.1128/jvi.63.5.1945-1950.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M. Y., Goldberg J., Coates L., Mason W., Gerin J., Taylor J. Molecular cloning of hepatitis delta virus RNA from an infected woodchuck liver: sequence, structure, and applications. J Virol. 1988 Jun;62(6):1855–1861. doi: 10.1128/jvi.62.6.1855-1861.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M. Y., Sharmeen L., Dinter-Gottlieb G., Taylor J. Characterization of self-cleaving RNA sequences on the genome and antigenome of human hepatitis delta virus. J Virol. 1988 Dec;62(12):4439–4444. doi: 10.1128/jvi.62.12.4439-4444.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luo G. X., Chao M., Hsieh S. Y., Sureau C., Nishikura K., Taylor J. A specific base transition occurs on replicating hepatitis delta virus RNA. J Virol. 1990 Mar;64(3):1021–1027. doi: 10.1128/jvi.64.3.1021-1027.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharmeen L., Kuo M. Y., Taylor J. Self-ligating RNA sequences on the antigenome of human hepatitis delta virus. J Virol. 1989 Mar;63(3):1428–1430. doi: 10.1128/jvi.63.3.1428-1430.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sureau C., Taylor J., Chao M., Eichberg J. W., Lanford R. E. Cloned hepatitis delta virus cDNA is infectious in the chimpanzee. J Virol. 1989 Oct;63(10):4292–4297. doi: 10.1128/jvi.63.10.4292-4297.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trono D., Feinberg M. B., Baltimore D. HIV-1 Gag mutants can dominantly interfere with the replication of the wild-type virus. Cell. 1989 Oct 6;59(1):113–120. doi: 10.1016/0092-8674(89)90874-x. [DOI] [PubMed] [Google Scholar]
- Wang K. S., Choo Q. L., Weiner A. J., Ou J. H., Najarian R. C., Thayer R. M., Mullenbach G. T., Denniston K. J., Gerin J. L., Houghton M. Structure, sequence and expression of the hepatitis delta (delta) viral genome. Nature. 1986 Oct 9;323(6088):508–514. doi: 10.1038/323508a0. [DOI] [PubMed] [Google Scholar]
- Weiner A. J., Choo Q. L., Wang K. S., Govindarajan S., Redeker A. G., Gerin J. L., Houghton M. A single antigenomic open reading frame of the hepatitis delta virus encodes the epitope(s) of both hepatitis delta antigen polypeptides p24 delta and p27 delta. J Virol. 1988 Feb;62(2):594–599. doi: 10.1128/jvi.62.2.594-599.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wejksnora P. J., Haber J. E. Ribonucleoprotein particle appearing during sporulation in yeast. J Bacteriol. 1978 Apr;134(1):246–260. doi: 10.1128/jb.134.1.246-260.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]