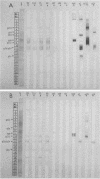
Abstract
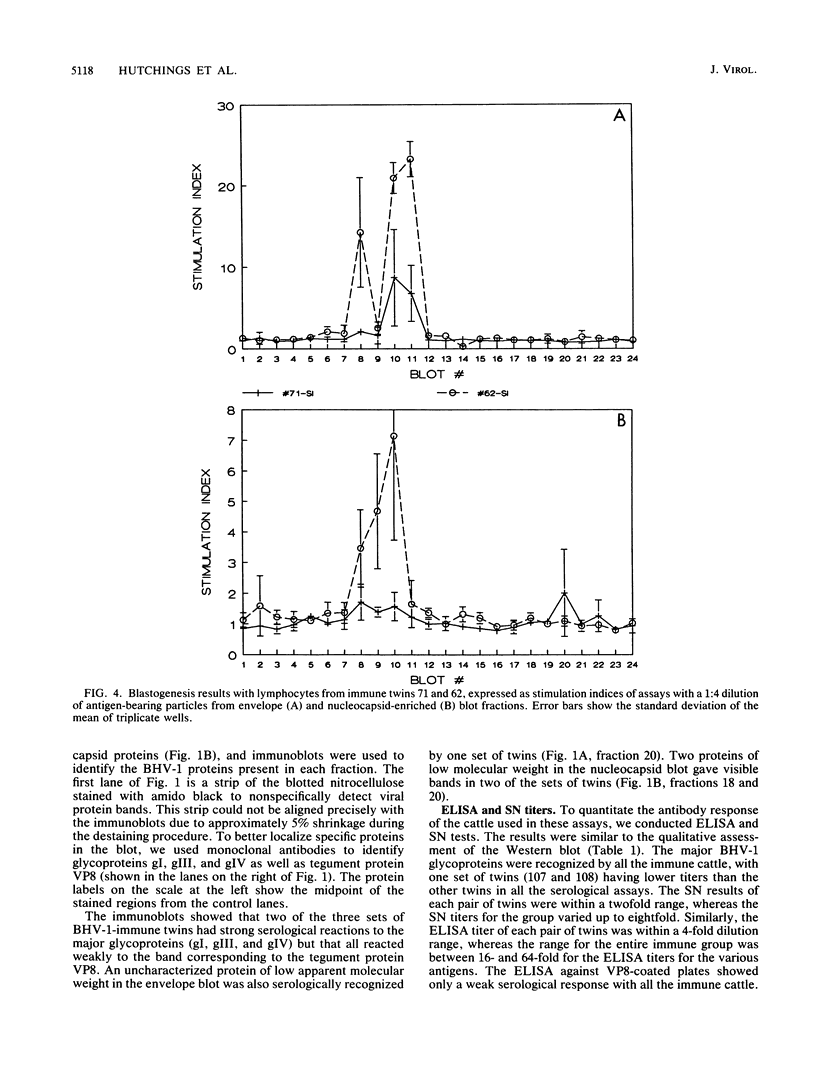
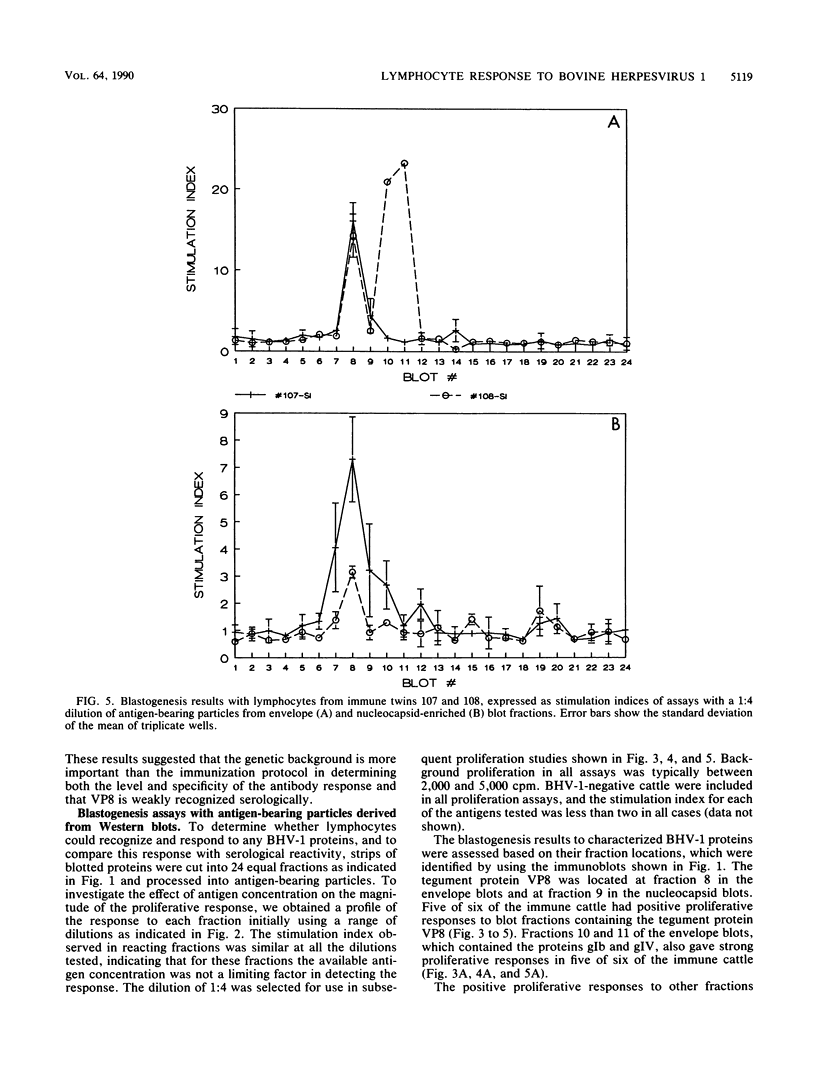
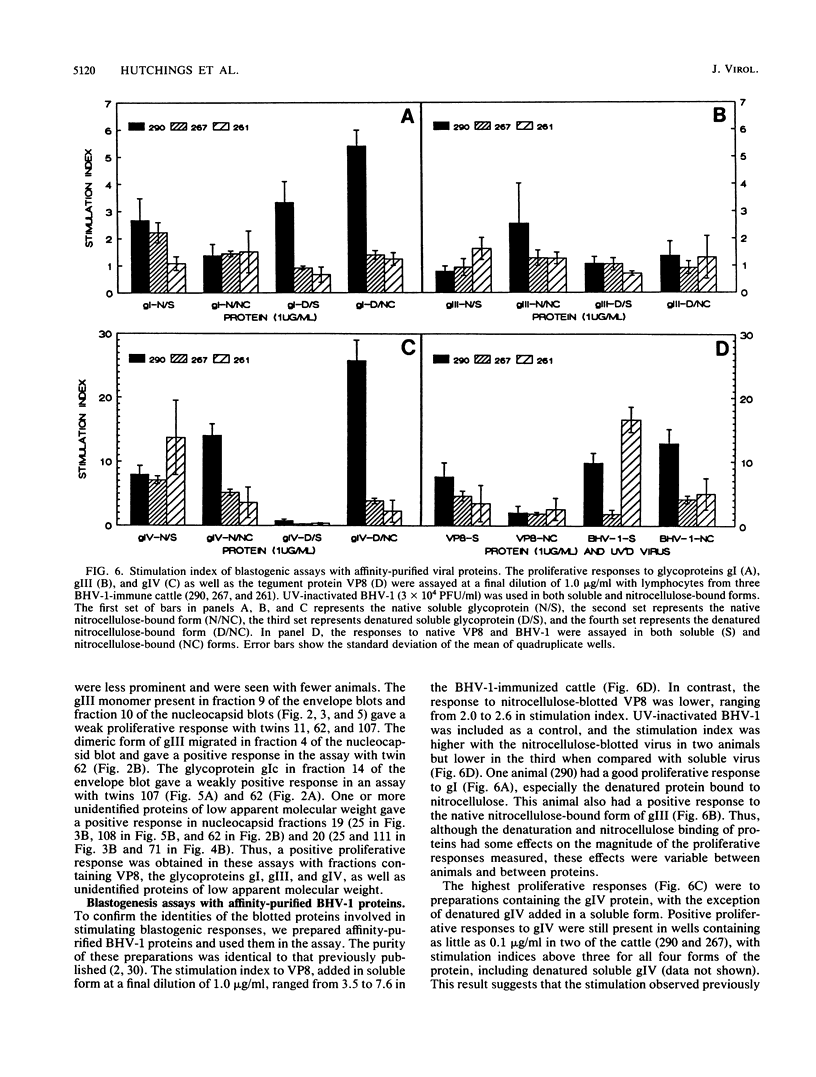
The immune response to bovine herpesvirus 1 (BHV-1) infection can protect cattle from subsequent challenge with the virus. This protection involves a variety of defensive strategies, and the activation of most of these defenses requires the recognition of viral proteins by the cellular immune system. To identify some of the BHV-1 proteins recognized by T lymphocytes, we measured in vitro proliferative responses to individual proteins. Viral proteins were separated by gel electrophoresis followed by Western immunoblotting, and immunoblots were evaluated for serological reactions. Unstained blotted fractions were processed into antigen-bearing particles for analysis in blastogenesis assays. Purified BHV-1 proteins obtained by immunoadsorbent chromatography were processed and included for comparison in both enzyme-linked immunosorbent and proliferation assays. The tegument protein VP8 and the glycoprotein gIV appeared to be the antigens which most consistently stimulated the proliferation of lymphocytes from BHV-1-immunized animals. Positive blastogenic responses were also detected to gI, gIII, and to one or more uncharacterized, low-molecular-weight proteins in some of the cattle tested. These results indicate that T-lymphocyte proliferative responses to BHV-1 proteins are detectable in immune cattle and may be important in protection from BHV-1 infection.
Full text
PDF
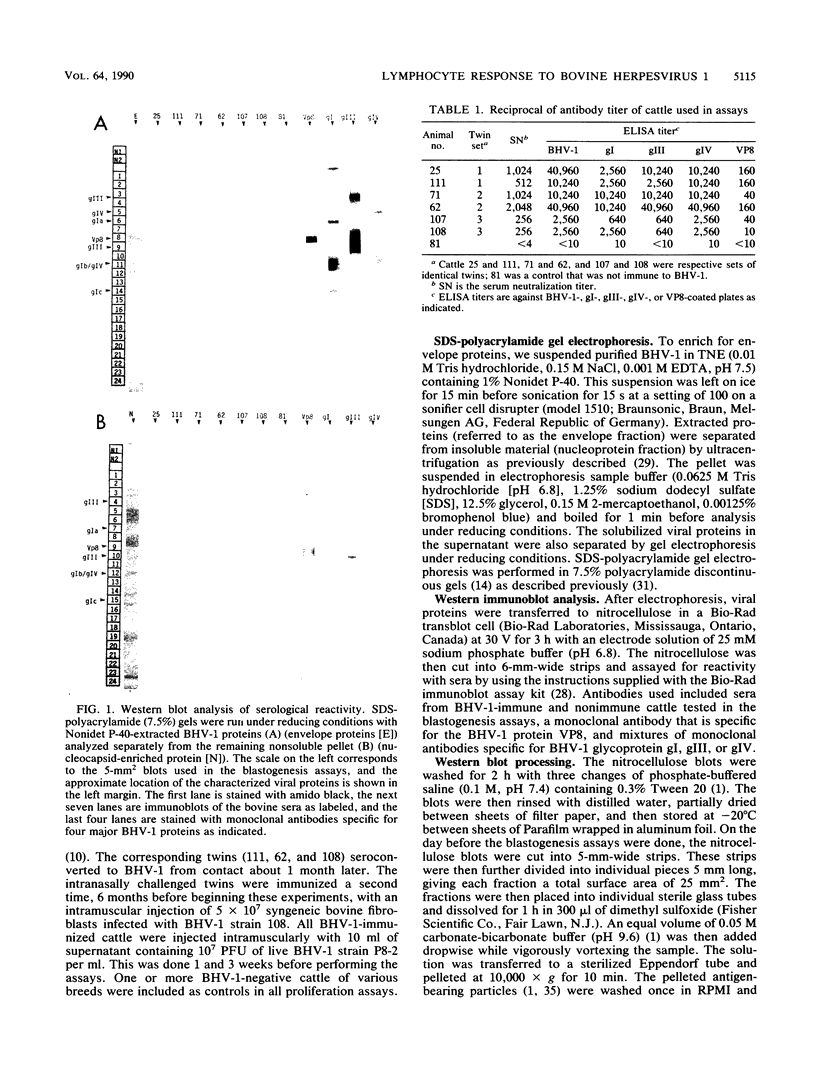
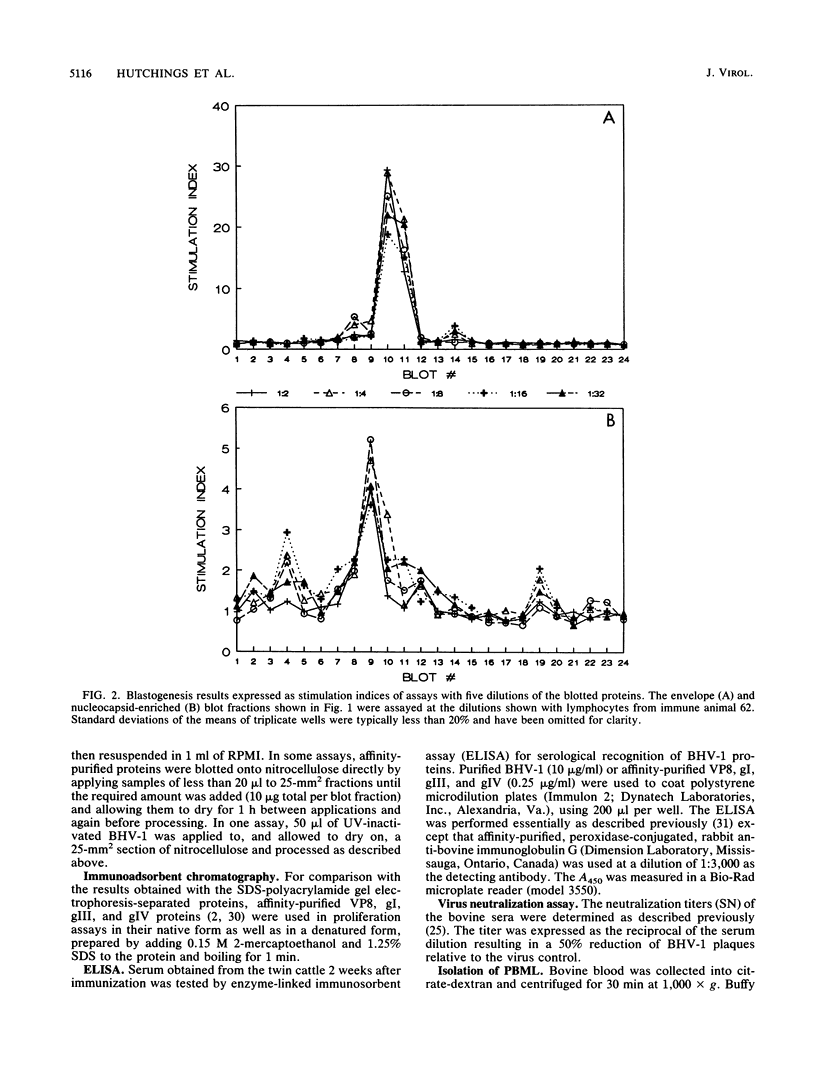
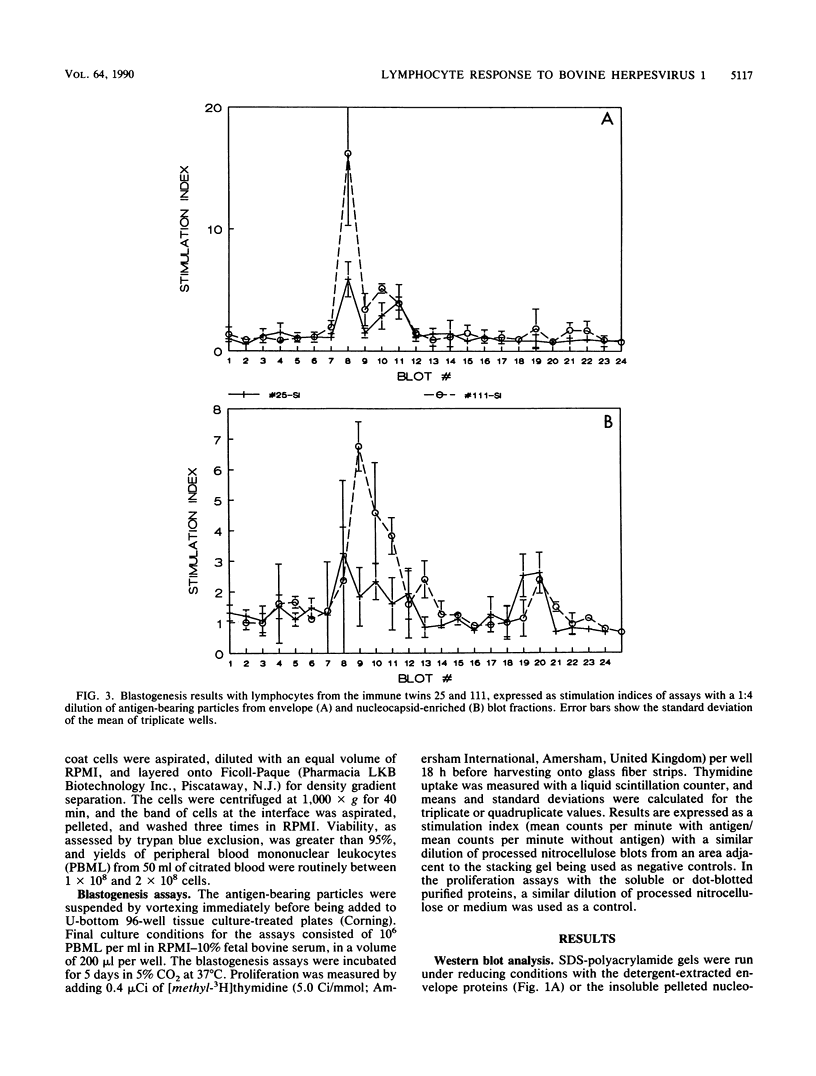


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Filley E., Steele J., Rook G. A. A simple new method for using antigens separated by polyacrylamide gel electrophoresis to stimulate lymphocytes in vitro after converting bands cut from Western blots into antigen-bearing particles. J Immunol Methods. 1987 Apr 2;98(1):5–10. doi: 10.1016/0022-1759(87)90429-7. [DOI] [PubMed] [Google Scholar]
- Babiuk L. A., L'Italien J., van Drunen Littel-van den Hurk S., Zamb T., Lawman J. P., Hughes G., Gifford G. A. Protection of cattle from bovine herpesvirus type I (BHV-1) infection by immunization with individual viral glycoproteins. Virology. 1987 Jul;159(1):57–66. doi: 10.1016/0042-6822(87)90347-3. [DOI] [PubMed] [Google Scholar]
- Babiuk L. A., Rouse B. T. Interactions between effector cell activity and lymphokines: implications for recovery from herpesvirus infections. Int Arch Allergy Appl Immunol. 1978;57(1):62–73. doi: 10.1159/000232085. [DOI] [PubMed] [Google Scholar]
- Babiuk L. A., Wardley R. C., Rouse B. T. Defense mechanisms against bovine herpesvirus: relationship of virus-host cell events to susceptibility to antibody-complement cell lysis. Infect Immun. 1975 Nov;12(5):958–963. doi: 10.1128/iai.12.5.958-963.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacklaws B. A., Nash A. A., Darby G. Specificity of the immune response of mice to herpes simplex virus glycoproteins B and D constitutively expressed on L cell lines. J Gen Virol. 1987 Apr;68(Pt 4):1103–1114. doi: 10.1099/0022-1317-68-4-1103. [DOI] [PubMed] [Google Scholar]
- Campos M., Ohmann H. B., Hutchings D., Rapin N., Babiuk L. A., Lawman M. J. Role of interferon-gamma in inducing cytotoxicity of peripheral blood mononuclear leukocytes to bovine herpesvirus type 1 (BHV-1)-infected cells. Cell Immunol. 1989 Apr 15;120(1):259–269. doi: 10.1016/0008-8749(89)90193-7. [DOI] [PubMed] [Google Scholar]
- Collins J. K., Butcher A. C., Riegel C. A. Immune response to bovine herpes herpesvirus type 1 infections: virus-specific antibodies in sera from infected animals. J Clin Microbiol. 1985 Apr;21(4):546–552. doi: 10.1128/jcm.21.4.546-552.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick D. R., Zamb T., Parker M. D., van Drunen Littel-van den Hurk S., Babiuk L. A., Lawman M. J. Expression of bovine herpesvirus 1 glycoproteins gI and gIII in transfected murine cells. J Virol. 1988 Nov;62(11):4239–4248. doi: 10.1128/jvi.62.11.4239-4248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson C. A., Schlesinger J. J., Barrett A. D. Prospects for a virus non-structural protein as a subunit vaccine. Vaccine. 1988 Feb;6(1):7–9. doi: 10.1016/0264-410x(88)90004-7. [DOI] [PubMed] [Google Scholar]
- Griebel P. J., Qualtiere L., Davis W. C., Gee A., Bielefeldt Ohmann H., Lawman M. J., Babiuk L. A. T lymphocyte population dynamics and function following a primary bovine herpesvirus type-1 infection. Viral Immunol. 1987;1(4):287–304. doi: 10.1089/vim.1987.1.287. [DOI] [PubMed] [Google Scholar]
- Hughes G., Babiuk L. A., van Drunen Littel-van den Hurk S. Functional and topographical analyses of epitopes on bovine herpesvirus type 1 glycoprotein IV. Arch Virol. 1988;103(1-2):47–60. doi: 10.1007/BF01319808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel B. A., Marshall R. L., Letchworth G. J., 3rd Epitope specificity and protective efficacy of the bovine immune response to bovine herpesvirus-1 glycoprotein vaccines. Vaccine. 1988 Aug;6(4):349–356. doi: 10.1016/0264-410x(88)90182-x. [DOI] [PubMed] [Google Scholar]
- Kuwano K., Scott M., Young J. F., Ennis F. A. Active immunization against virus infections due to antigenic drift by induction of crossreactive cytotoxic T lymphocytes. J Exp Med. 1989 Apr 1;169(4):1361–1371. doi: 10.1084/jem.169.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., O'Hehir R. E., Young D. B. The use of nitrocellulose immunoblots for the analysis of antigen recognition by T lymphocytes. J Immunol Methods. 1988 May 25;110(1):1–10. doi: 10.1016/0022-1759(88)90076-2. [DOI] [PubMed] [Google Scholar]
- Lawman M. J., Courtney R. J., Eberle R., Schaffer P. A., O'Hara M. K., Rouse B. T. Cell-mediated immunity to herpes simplex virus: specificity of cytotoxic T cells. Infect Immun. 1980 Nov;30(2):451–461. doi: 10.1128/iai.30.2.451-461.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum M. A., Reed D. E. Identification of bovine herpesvirus-1 polypeptides involved in serum neutralization. Vet Microbiol. 1986 Mar;11(3):213–220. doi: 10.1016/0378-1135(86)90024-6. [DOI] [PubMed] [Google Scholar]
- Marshall R. L., Rodriguez L. L., Letchworth G. J., 3rd Characterization of envelope proteins of infectious bovine rhinotracheitis virus (bovine herpesvirus 1) by biochemical and immunological methods. J Virol. 1986 Mar;57(3):745–753. doi: 10.1128/jvi.57.3.745-753.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menanteau-Horta A. M., Ames T. R., Johnson D. W., Meiske J. C. Effect of maternal antibody upon vaccination with infectious bovine rhinotracheitis and bovine virus diarrhea vaccines. Can J Comp Med. 1985 Jan;49(1):10–14. [PMC free article] [PubMed] [Google Scholar]
- Misra V., Blumenthal R. M., Babiuk L. A. Proteins Specified by bovine herpesvirus 1 (infectious bovine rhinotracheitis virus). J Virol. 1981 Nov;40(2):367–378. doi: 10.1128/jvi.40.2.367-378.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins D. L., Lai M. Z., Smith J. A., Gefter M. L. Identical peptides recognized by MHC class I- and II-restricted T cells. J Exp Med. 1989 Jul 1;170(1):279–289. doi: 10.1084/jem.170.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse B. T., Babiuk L. A. Host defense mechanisms against infectious bovine rhinotracheitis virus. II. Inhibition of viral plaque formation by immune peripheral blood lymphocytes. Cell Immunol. 1975 May;17(1):43–56. doi: 10.1016/s0008-8749(75)80005-0. [DOI] [PubMed] [Google Scholar]
- Rouse B. T., Babiuk L. A. Host defense mechanisms against infectious bovine rhinotracheitis virus: in vitro stimulation of sensitized lymphocytes by virus antigen. Infect Immun. 1974 Oct;10(4):681–687. doi: 10.1128/iai.10.4.681-687.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse B. T., Babiuk L. A. Mechanisms of recovery from Herpesvirus infections -a review. Can J Comp Med. 1978 Oct;42(4):414–427. [PMC free article] [PubMed] [Google Scholar]
- Rouse B. T., Babiuk L. A. The direct antiviral cytotoxicity by bovine lymphocytes is not restricted by genetic incompatibility of lymphocytes and target cells. J Immunol. 1977 Feb;118(2):618–624. [PubMed] [Google Scholar]
- Rouse B. T., Wardley R. C., Babiuk L. A. The role of antibody dependent cytotoxicity in recovery from herpesvirus infections. Cell Immunol. 1976 Mar 1;22(1):182–186. doi: 10.1016/0008-8749(76)90019-8. [DOI] [PubMed] [Google Scholar]
- Scott N. A., Whalley J. M., Mattick J. S., Underwood P. A., Aboud L., Williams K. L., Kirkland P. Identification of major antigenic proteins of bovine herpesvirus 1 and their correlation with virus neutralizing activity. Vet Microbiol. 1988 Feb;16(2):109–121. doi: 10.1016/0378-1135(88)90035-1. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Fernandez-Botran R., Myers C. D., Sanders V. M. Cellular interactions in the humoral immune response. Adv Immunol. 1989;45:1–105. doi: 10.1016/s0065-2776(08)60692-6. [DOI] [PubMed] [Google Scholar]
- Young D. B., Lamb J. R. T lymphocytes respond to solid-phase antigen: a novel approach to the molecular analysis of cellular immunity. Immunology. 1986 Oct;59(2):167–171. [PMC free article] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., Babiuk L. A. Antigenic and immunogenic characteristics of bovine herpesvirus type-1 glycoproteins GVP 3/9 and GVP 6/11a/16, purified by immunoadsorbent chromatography. Virology. 1985 Jul 15;144(1):204–215. doi: 10.1016/0042-6822(85)90318-6. [DOI] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., Babiuk L. A. Polypeptide specificity of the antibody response after primary and recurrent infection with bovine herpesvirus 1. J Clin Microbiol. 1986 Feb;23(2):274–282. doi: 10.1128/jcm.23.2.274-282.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., Babiuk L. A. Synthesis and processing of bovine herpesvirus 1 glycoproteins. J Virol. 1986 Aug;59(2):401–410. doi: 10.1128/jvi.59.2.401-410.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., Zamb T., Babiuk L. A. Synthesis, cellular location, and immunogenicity of bovine herpesvirus 1 glycoproteins gI and gIII expressed by recombinant vaccinia virus. J Virol. 1989 May;63(5):2159–2168. doi: 10.1128/jvi.63.5.2159-2168.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., van den Hurk J. V., Gilchrist J. E., Misra V., Babiuk L. A. Interactions of monoclonal antibodies and bovine herpesvirus type 1 (BHV-1) glycoproteins: characterization of their biochemical and immunological properties. Virology. 1984 Jun;135(2):466–479. doi: 10.1016/0042-6822(84)90201-0. [DOI] [PubMed] [Google Scholar]