Abstract
The Ca2+ channel α1A-subunit is a voltage-gated, pore-forming membrane protein positioned at the intersection of two important lines of research: one exploring the diversity of Ca2+ channels and their physiological roles, and the other pursuing mechanisms of ataxia, dystonia, epilepsy, and migraine. α1A-Subunits are thought to support both P- and Q-type Ca2+ channel currents, but the most direct test, a null mutant, has not been described, nor is it known which changes in neurotransmission might arise from elimination of the predominant Ca2+ delivery system at excitatory nerve terminals. We generated α1A-deficient mice (α1A−/−) and found that they developed a rapidly progressive neurological deficit with specific characteristics of ataxia and dystonia before dying ≈3–4 weeks after birth. P-type currents in Purkinje neurons and P- and Q-type currents in cerebellar granule cells were eliminated completely whereas other Ca2+ channel types, including those involved in triggering transmitter release, also underwent concomitant changes in density. Synaptic transmission in α1A−/− hippocampal slices persisted despite the lack of P/Q-type channels but showed enhanced reliance on N-type and R-type Ca2+ entry. The α1A−/− mice provide a starting point for unraveling neuropathological mechanisms of human diseases generated by mutations in α1A.
The α1A-subunit, the most abundant α1-subunit in vertebrate brain (1), mediates Ca2+ influx across presynaptic and somatodendritic membranes, thereby triggering fast neurotransmitter release and other key neuronal responses (2–5). Because of its high expression levels in the brain, the α1A-subunit was the first representative of its subclass to be isolated by cDNA cloning (1, 6). This predominantly neuronal subclass also includes α1B (N-type Ca2+ channel) and α1E [possibly R-type Ca2+ channel (7–9)] and is referred to as ABE or CaV2. There is no information to date on the behavioral or electrophysiological consequences of deleting a member of the ABE subfamily.
α1A Transcripts are widely distributed in rat (10) and human brain (11), most prominently in cell body layers in cerebellum and hippocampus. At the subcellular level, α1A immunoreactivity has been found in cell bodies, dendrites, and presynaptic terminals (12). Less clear has been the role of α1A in supporting Ca2+ channel components defined by biophysical and pharmacological criteria. In either Xenopus oocytes (13, 14) or HEK293 cells (15), expression of α1A-subunits along with ancillary α2/δ- and β-subunits generated currents with properties closely resembling the Q-type current found in cerebellar granule cells (8) and much less the P-type current first described in cerebellar Purkinje neurons by Llinás and colleagues (16, 17). Unlike native P-type channels (18), the expressed currents showed pronounced inactivation during sustained depolarizations and responded to ω-agatoxin IVA (ω-Aga-IVA) at half-blocking doses of ≈100 nM, not ≈1 nM (13). Various explanations for the discrepancies have been advanced, including differences in pore-forming subunits (19), diversity of β-subunits (20, 21), and alternative splicing of α1A transcripts (22, 23). Whether any of these proposals fully account for the properties of native P-type channels remains uncertain (22, 24), although Gillard et al. (25) showed that α1A antisense oligonucleotides reduce P-type current in cerebellar Purkinje cells (see also refs. 26 and 27). Bourinet et al. (22) demonstrated that splice variations account for most of the differences between prototypic P- and Q-type behavior but carefully pointed out that no variant as yet has fully replicated the characteristics of P-type currents in Purkinje neurons (16, 18).
Several spontaneous mutations in α1A with diverse phenotypes have been found in mice (28, 29) and humans (30, 31). Characterization of these alleles has indicated that none of them are null mutations: currents attributed to α1A were partially diminished (32–36) or even increased (35, 36). To unravel the underlying mechanisms of the diverse neuropathological defects generated by these mutations, it is essential to determine the status of animals in which α1A-dependent channel activity has been eliminated completely.
Methods
Animals.
The mice used for Southern, Northern, and Western blot analyses were F2 progeny derived from intercrosses of the heterozygotes in the F1 (129/sv × C57BL/6J) genetic background. Other experiments were done with mice that had 129/sv genetic background. All animal handling was in accordance with guidelines of the Animal Care and Use Committee.
Targeting Vector Construction.
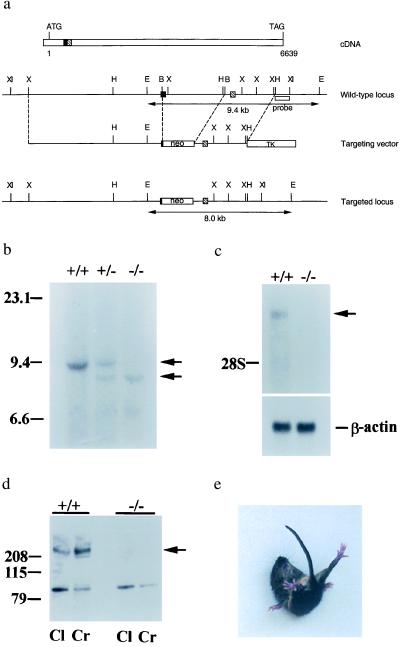
The strategy for vector construction was as described (37). A genomic clone that had two exons that encode the nucleotide sequences 401–493 and 494–646 bp from the initiation codon of cDNA was isolated by using a 410-bp rat α1A cDNA probe, located at the 5′ region of the calcium channel, mapped by various enzymes, and used to make the targeting vector. As shown in Fig. 1a, a BalI fragment that includes part of the exon and intron was deleted and replaced with the neo cassette [PGK promoter-neo-PGK poly(A)] for gene disruption and positive selection. The TK [PGK promoter–thymidine kinase-PGK poly(A)] cassette was attached to the end of the 3′ homology region for negative selection. The final construct had a 7.5-kb 5′ homology and a 2.75-kb 3′ homology to the calcium channel α1A genomic locus.
Figure 1.
Characterization of the mouse Ca2+ channel α1A null mutation. (a) The start and the stop codons are indicated in the cDNA map. The solid box and the hatched box represent the corresponding exons in the genomic DNA locus. Double arrowhead lines under the wt and the targeted locus represent the expected fragments when digested with EcoRI and hybridized with the probe (open box underneath the wt locus). B, BalI; E, EcoRI; H, HindIII; X, XbaI; XI, XhoI. (b) Southern blot analysis of offspring derived from intercrosses of calcium channel α1A heterozygous parents. The 9.4-kb fragment is the wt allele and the 9.0-kb fragment is the targeted allele. +/+, wt; +/−, heterozygote; −/−, mutant. (c) Northern blot analysis of brain tissues. One-microgram aliquots of poly(A) RNA from wt and −/− animals were probed with a 410-bp fragment from the 5′ region of rat brain α1A cDNA. The lower bands are β-actin mRNA, used as a control. (d) Western blot analysis of membrane fractions from cerebral (Cr) and cerebellar (Cl) cortices of wt and α1A−/− mice. The band observed near 100 kDa in α1A−/− is unlikely to correspond to the glycosylated, 95-kDa short form of α1A (19) because it was eliminated after purification by affinity to wheat germ-agglutinin-Sepharose. Furthermore, no reverse transcription–PCR products were obtained from α1A−/− mRNA with various primer sets targeting the first half of the channel, in contrast to the finding of PCR products of correct length when wt mRNA was tested (see Methods). (e) An α1A mutant mouse attempting to right itself during a falling episode.
Immunohistochemistry.
P 21 mice were anesthetized with Avertin (1.25% tribromoethanol/amyl alcohol) by i.p. injection and perfused transcardially with PBS followed by 4% paraformaldehyde. The brain was postfixed overnight in the same fixative. Serial sagittal or coronal sections were cut at 50 μm with a vibratome. Sections were processed free-floating with the avidin-biotin-peroxidase complex method and stained with toluidine blue. Tissue sections were treated successively with 0.3% Triton X-100 (15 min), 1 μg/ml Proteinase K (30 min), and 3% H2O2 in PBS (3 min) and washed (three times) in PBS (pH 7.4). Samples were treated for 1 hr with PBS supplemented with 3% normal goat serum (Sigma) and incubated overnight at 4°C with antibodies against Calbindin (1:3,000; Sigma) or tyrosine hydroxylase (1:300; Chemicon). Sections were washed with PBS and incubated for 2 hr at room temperature with goat anti-rabbit biotinylated secondary antibody (1:200) in PBS plus 3% goat serum. Sections were incubated with the avidin-biotin complex for 40 min at room temperature and developed with a diaminobenzidine solution (Vector).
Ca2+ Channel Current Recordings.
Cultured cerebellar granule cells and acutely dissociated Purkinje neurons were prepared as described (8, 18). Granule cells were obtained from 5-day-old pups and kept in culture for 5–9 days in vitro before use. Purkinje neurons were obtained from 12- to 15-day-old animals. Whole-cell recordings were obtained by using standard protocols. Current traces were corrected for linear leak and capacitative currents with on-line −P/4 trace subtraction. Mean cell capacitance for each experimental group was (in pF): granule cells (GC): 5.4 ± 0.6 (+/+, n = 14), 4.5 ± 0.3 (+/−, n = 8), and 5.6 ± 1.4 (−/−, n = 13); Purkinje neurons (PN): 13.5 ± 0.9 (+/+, n = 23), 16.0 ± 1.6 (+/−, n = 15), and 16.1 ± 1.6 (−/−, n = 14). Series resistance was 6.9 ± 0.4 MΩ (n = 35) for granule cells and 2.4 ± 0.2 MΩ (n = 45) for Purkinje neurons (typically compensated ≈75%). Gigaseals were obtained in Tyrode solution, and whole-cell recordings were made in extracellular solution. Solution composition was, for Tyrode, 150 mM NaCl/4 mM KCl/2 mM CaCl2/2 mM MgCl2/10 mM Hepes/10 mM glucose, pH = 7.4. Extracellular solution consisted of 140 or 160 mM tetraethylammonium-Cl, 10 or 2 mM BaCl2 (for GC and PN, respectively), 10 mM Hepes-CsOH, 10 mM glucose, 0.001 mM tetrodotoxin, and 1 mg/ml cytochrome c, pH 7.4, 300 mOsM. Intracellular solution consisted of 108 mM cesium-methanesulfonate, 4.5 mM MgCl2, 1 mM EGTA, 9 mM Hepes-tetraethylammonium-OH, 4 mM ATP-Na, 1 mM GTP-Na, and 15 mM creatine phosphate, pH 7.3, 290 mOsM. Corrections for liquid junction potentials were made. All experiments were performed at room temperature (22–24°C). Data are reported as the mean ± SEM. Statistical significance was assessed by using Student's t test.
Neurotransmission in Brain Slices.
Transverse hippocampal slices (400 μm) were obtained by standard methods (38) from 21- to 30-day-old mice of either sex. Mice were anesthetized with isofluorane and decapitated, and the hippocampi were dissected quickly in cold Krebs buffer containing 130 mM NaCl, 2.5 mM KCl, 1.3 mM MgCl2, 2.0 mM CaCl2, 1.0 mM NaH2PO4, 26.2 mM NaHCO3, and 11.0 mM glucose and gassed with 95% O2 and 5% CO2. Slices were placed in a humidified holding chamber that was gassed continually with the O2/CO2 mixture on a piece of filter paper subfused with Krebs and allowed to recover at least 60 min before attempting recording. All slice recordings were made at room temperature (24°C) with slices submerged and superfused continuously (≈1 ml/min) with Krebs pregassed with the O2/CO2 mixture. Synaptic transmission was elicited every 30 s by stimulation of the Schaffer collateral and commissural pathways. Field excitatory postsynaptic potentials (EPSPs) were recorded with 1- to 3-MΩ pipettes filled with 3 M NaCl. Responses were analyzed by measuring the maximal slope of the EPSP. Averaged data are reported as mean ± SEM, and n represents the number of independent pathways, usually two per slice. Drugs were applied by addition to the superfusion reservoir. Responses were acquired and analyzed by using lab-specific programs written in labview (National Instruments, Austin, TX).
Results
α1A Knockout Animals.
As a direct approach to understanding the physiological contributions of the α1A-subunit to Ca2+ channel currents and synaptic transmission, and to assess its neuropathological effects, we generated α1A null mutants by gene targeting (Fig. 1a). Mice heterozygous for the α1A gene showed no apparent abnormality and were fertile. Heterozygous mice were intercrossed and their offspring were analyzed by Southern blotting (Fig. 1b) and PCR (data not shown). The α1A null mutation was confirmed by Northern blotting (Fig. 1c), reverse transcription–PCR (data not shown), and Western blotting (Fig. 1d). Of the offspring derived from heterozygote intercrosses, 32 (25%) were wild type (wt), 64 (51%) were heterozygotes, and 30 (24%) were homozygotes (α1A−/−). This typical Mendelian ratio for the α1A mutation was consistent with a normal embryonic development; if severe deficits existed in the α1A−/− mice, they were not detected.
Surprisingly, the α1A−/− mice initially were viable despite the demonstrated importance of the α1A-subunit for neurotransmission (39). The animals appeared healthy until ≈10 days after birth, when they developed a tendency to lose balance when walking and then roll over onto their backs. This ataxia and the resulting falls became increasingly common with age, such that by P20 the animals were unable to walk at all. At all ages, falls were followed by sustained, twisting movements of the trunk and limbs, which, in younger mice, often resulted in the animal righting itself. However, in the older animals, the movements were more prolonged and less successful, possibly consistent with dystonia or weakness (Fig. 1e). These prolonged episodes often were interrupted by extensor spasms of the hind limbs. α1A−/− mice, like the spontaneously occurring mutant mice, tottering (tg) and leaner (tgla), exhibited brief episodes when all movement stopped. These attacks have been shown to reflect absence seizures in tg and tgla mice (SI). The null mutant mice were smaller than wt littermates by the time of death in the fourth week of life, in contrast to the spontaneously occurring α1A mutant mice, which survive to adulthood when carefully nourished. The α1A mutant pups died despite an intact suckling reflex, even when littermates were removed to avoid competition, and despite hand-feeding with human infant formula.
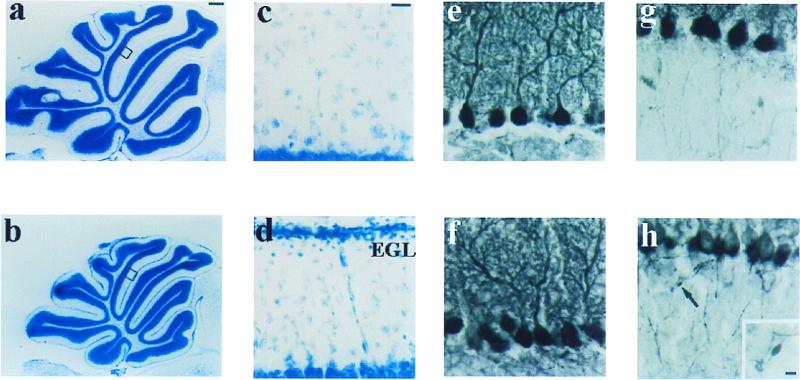
The cerebella of the mutants were smaller than those of their wt littermates but showed well developed patterns of cerebellar foliation (Fig. 2 a and b). At P21, an external granule cell layer was still present in the mutants (Fig. 2d), whereas it had completed its migration in wt cerebellum (Fig. 2c). The Purkinje cell monolayer and granule cell layers developed normally, with well differentiated dendritic arbors and no detectable deficit in the number of neurons (Fig. 2 e and f). However, immunostaining with anticalbindin antibody revealed Purkinje cell axons with abundant focal axonal swellings in the mutants (Fig. 2h), whereas this rarely occurred in wt (Fig. 2g).
Figure 2.
Histological and immunohistochemical analyses of brain in wt and α1A−/− mouse at P21. (a and b) Toluidine blue-stained sagittal sections of cerebellar vermis. (c and d) Higher magnification of areas in a and b. The mutant showed the persistence of an external granule cell layer, indicating a delay or deficit in granule cell migration (d). (e– h) Cerebellar cortex immunostained with anticalbindin antibody. (e and f) Dendritic trees of Purkinje cells. (g and h) Axons of Purkinje cells in granule cell layer. Arrow indicates focal axonal swellings that frequently are found in α1A−/− (h Inset). EGL, external granule cell layer. [Bars = 400 μm (a and b), 25 μm (c– h), and 5 μm (h Inset).]
We also examined the distribution of tyrosine hydroxylase (TH) because abnormal expression has been reported (40) in the spontaneous α1A mutants tottering and leaner (28). At P21, the appearance of TH-immunoreactive neurons in brains of α1A−/− mice was largely similar to that of wt littermates: TH expression was sustained in the major catecholaminergic nuclei (substantia nigra, ventral tegmental area, and locus ceruleus) and transient in posterior cerebellar Purkinje cells. The main difference was that numerous TH-immunoreactive neurons appeared in deeper cortical layers of α1A−/− mice whereas very few could be observed in wt, consistent with a developmental delay in the null mutants.
Changes in Ca2+ Channel Currents.
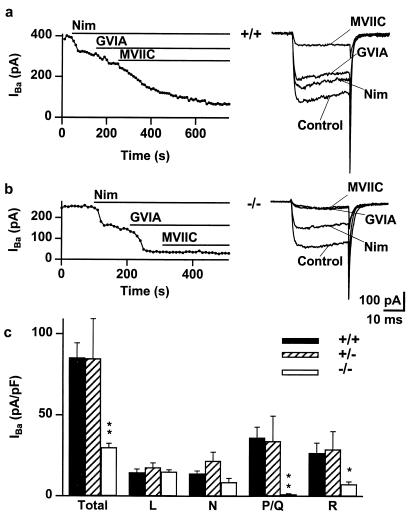
α1A is abundant in the cerebellum (1, 10, 12, 14), particularly in the Purkinje neurons (16–18) and granule cells (8) in which, respectively, P- and Q-type Ca2+ channel currents first were defined. Both kinds of neurons were used to study the effect of deleting the α1A-subunit. In cerebellar granule cells, the array of Ca2+ channel currents supported by Ba2+ as charge carrier was defined by successive applications of specific blockers, nimodipine (10 μM) for L-type, ω-CTx-GVIA (1 μM) for N-type (41), and ω-CTx-MVIIC (5 μM) for P/Q-type (41), leaving a residual R-type component (8). Fig. 3 compares inward currents evoked by depolarizations from –90 mV to –10 mV in wt and α1A−/− neurons. In pooled data, total current density was reduced from 85.2 pA/pF (n = 14) in wt to 29.9 pA/pF (n = 14) in α1A−/− granule neurons, a decrease of 65% (P < 0.0001). The reduction in current in α1A−/− was accounted for by reductions in the contributions of ω-CTx-MVIIC-sensitive current (34.9 pA/pF, P < 0.002) and the residual R-type current (19.2 pA/pF, P < 0.02). The 97% reduction of the ω-CTx-MVIIC-sensitive current provided direct evidence that the α1A-subunit was essential for P/Q-type current as hypothesized previously (8, 26). The 72% reduction in R-type current was unexpected. That this fraction of R-type current was supported directly by α1A-subunits cannot be ruled out, but seems unlikely given the unresponsiveness of R-type current to antisense oligonucleotides against α1A (27). Another possibility is that the α1A-subunit may help control the expression of R-type channels or act as a positive modulator of their activity (50).
Figure 3.
P/Q-type calcium channels are absent and R-type calcium channels are diminished in α1A−/− cerebellar granule cells. (a) Peak IBa, activated by 30-ms depolarizations from –90 mV to –10 mV every 10 s, is plotted against time for an α1A+/+ neuron. The total current was reduced by the application of nimodipine (10 μM), ω-CTx-GVIA (1 μM), and ω-CTx-MVIIC (5 μM), which blocked the L-, N-, and P/Q-type components of the current, respectively. Representative traces are shown to the right of the graph. Ba2+ (10 mM) as charge carrier. (b) Peak IBa, activated as in a, is plotted against time for an α1A−/− neuron. (c) Histogram of total and individual channel type current density in α1A+/+ (solid), α1A+/− (hatched), and α1A−/− (open) granule cells. The total current density is reduced in α1A−/− neurons; this is accounted for by the absence of the P/Q-type channels and the reduction of R-type current. **, P < 0.002, *, P < 0.05.
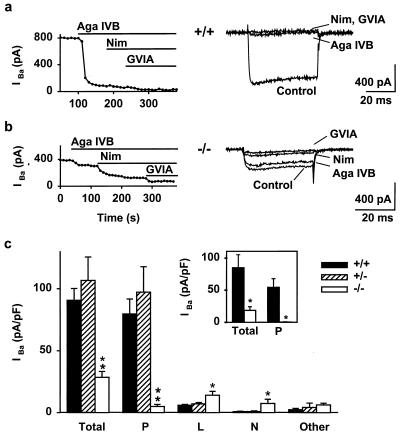
In cerebellar Purkinje neurons (Fig. 4c), Ca2+ current density was decreased from 90.7 ± 9.5 pA/pF in wt mice (n = 23) to 28.4 ± 4.8 pA/pF in null mutants (n = 14), a reduction of ≈70% (P < 10−7). Pharmacological characterization of currents was carried out by successive application of specific channel blockers (Fig. 4 a and b), and currents were activated every 10 s by depolarizations from –90 to –30 mV. The current blocked by ω-Aga-IVB (500 nM), an inhibitor of P/Q-type channels (42), was reduced much by deletion of α1A, dropping from 79.3 ± 12 pA/pF in wt Purkinje cells (n = 19) to 5.0 ± 1.7 pA/pF in the α1A−/− neurons (n = 14, P < 10−5). [The small residuum of ω-Aga IVB-sensitive component in the null mutants was traced to a slight effect of the agatoxin on L- or N-type currents (43); ω-Aga-IVB exerted no detectable effect if applied after L- and N-type currents had already been blocked (Fig. 4c Inset).] Interestingly, the current components quantified by further application of nimodipine (1 μM) and ω-CTx-GVIA (1 μM) were larger in the null mutant than in wt (Fig. 4c). Assessed in this way, the contribution of L-type channels increased from 6 ± 0.8 pA/pF in wt to 14 ± 3 pA/pF in α1A−/− (n = 17 and 12, P < 0.02) and that of N-type channels increased from 0.9 ± 0.2 pA/pF to 7.4 ± 3.4 pA/pF (n = 10 and 7, P < 0.002). A similar increase in L-type currents has been reported in the case of the tottering α1A mutant strain (44). The remaining Cd2+-sensitive currents were similar for both groups (P > 0.1). The overall conclusion is that P-type channel activity was eliminated completely by deletion of α1A and that expression of functional L- and N-type Ca2+ channels was augmented.
Figure 4.
P-type currents are eliminated and L- and N-type currents are augmented in Purkinje neurons from α1A−/−. (a and b) Pharmacological dissection of Ca2+ channel currents from α1A+/+ (a) and α1A−/− Purkinje neurons (b). Ba2+ (2 mM) as charge carrier. (Left) Response to successive application of 500 nM ω-Aga-IVB (purified native toxin), 1 μM nimodipine, and 1 μM ω-CTx-GVIA. (Right) Representative traces. (c) Current density distribution. Null mutants exhibited significantly smaller total current density than the controls. P-type currents were absent in α1A−/−. L- and N-type current densities were increased significantly in α1A−/−, whereas the other CdCl2-sensitive components did not change. **, P < 0.001; *, P < 0.05. (Inset) Total current (Total) and Aga IVB-sensitive current (“P”) after preblockade of L- and N-type currents with nimodipine and ω-CTx-GVIA. Note that Aga IVB showed no effect on the remaining currents in α1A−/−. Thus, the modest Aga IVB effect on the null mutant neuron currents (b) can be attributed to a slight inhibition of N- or L-type currents (43).
Alteration of Neurotransmission in Brain Slices.
The impact of the elimination of α1A on synaptic transmission was studied in area CA1 of hippocampal slices. Field EPSPs were evoked by stimulation of the Schaffer collateral and commissural pathways. Neurotransmission was well maintained with repetitive stimulation over hours, indicating that vesicle recycling was functional despite the absence of α1A (cf. ref. 45). Synaptic transmission in α1A−/− animals was unaffected by ω-Aga-IVB (1 μM) (data not shown) or nimodipine (10 μM) (Fig. 5f) but showed enhanced dependence on N-type Ca2+ channels. Blockade of N-type Ca2+ channels with ω-CTx-GVIA (1 μM), which halved the EPSP slope in wt mice (n = 10), nearly eliminated synaptic transmission in the α1A−/− animals (n = 8, P < 10−10) (Fig. 5a). To test the hypothesis that ablation of the α1A-subunit diminished Ca2+ influx at nerve terminals, we examined the effects of broadening the presynaptic action potential with the K+-channel blocker 4-aminopyridine (4-AP, 100 μM). Previous work has shown that pharmacological inhibition of presynaptic Ca2+ channels (by ω-Agatoxin, for example) magnifies the relative effect of 4-AP (46). Application of 4-AP (Fig. 5b) increased the EPSP slope by 302 ± 29% in the α1A−/− slices (n = 4), significantly greater then the 180 ± 27% enhancement in wt slices (n = 4, P < 0.05), consistent with the idea that Ca2+ influx at individual synaptic release sites had been diminished. Evidently, elimination of P/Q-type channels lowers the total number of channels available to support neurotransmission.
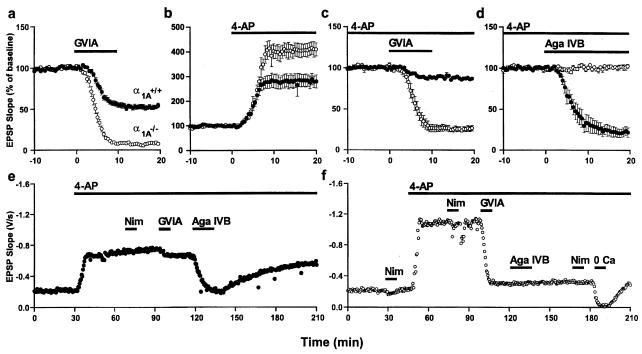
Figure 5.
Effects on synaptic transmission of altering Ca2+ influx in wt (●) and mutant (○) animals. (a) Blockade of N-type Ca2+ channels with ω-CTx-GVIA (1 μM) reduced the strength of synaptic transmission by half in wt but eliminated the EPSP in mutants. (b) Enhancement of Ca2+ influx by broadening action potentials with 4-AP (100 μM) increased the strength of synaptic transmission by 2-fold in wt and 3-fold in mutants. (c) After application of 4-AP, blockade of N-type Ca2+ channels considerably reduced synaptic strength in α1A−/− slices, with a much smaller effect in wt slices. (d) After application of 4-AP and ω-CTx-GVIA, blockade of P/Q-type Ca2+ channels with ω-Aga-IVB (1 μM) decreased the strength of synaptic transmission in wt by more than three-quarters but had no effect in mutants. (e and f) Representative responses to sequential application of Ca2+ channel blockers in the presence of 4-AP in wt (e) and α1A−/− (f) animals.
Additional tests of the pharmacological properties of synaptic transmission were performed in the presence of 4-AP to magnify disparities between α1A−/− and wt behavior. Blockade of N-type Ca2+ channels reduced synaptic strength by 13 ± 2% in wt (n = 4) and by 74 ± 3% in α1A−/− slices (n = 4, P < 10−5) (Fig. 5c). Further application of ω-Aga-IVB (1 μM) to block P/Q-type Ca2+ channels caused the strength of synaptic transmission to decrease by 78 ± 5% (n = 4) in wt but produced no additional decrease in α1A−/− (n = 4, P < 10−4) (Fig. 5d). In both wt (Fig. 5e) and α1A−/− animals (Fig. 5f), a significant component of synaptic transmission persisted even after blockade of N- and P/Q-type channels. This was not due to L-type channels, inasmuch as nimodipine failed to reduce the remaining transmission. Nonetheless, the residual transmission could be abolished reversibly by removal of external Ca2+. Thus, in the presence of K+ channel blockade, synaptic transmission can be supported by a voltage-gated Ca2+ channel that is neither L-, N-, nor P/Q-type (47, 48). Further studies are required to assess the degree to which this other Ca2+ channel type participates in normal synaptic transmission with and without the contributions of the α1A-subunit.
Discussion
Our experiments provided definitive evidence that the α1A-subunit generates both P- and Q-type currents, in agreement with previous work (22, 25, 27). This supports the common practice of grouping α1A gene products together with the generic label “P/Q-type,” recognizing that considerable diversity within this category is generated by splice variations (22, 23) among other factors. The absence of P/Q-type currents was associated with changes in other current components relative to wt, including elevation of L- and N-type current density in cerebellar Purkinje cell bodies and reduction of R-type current density in cerebellar granule cell bodies. These alterations were largely unanticipated based on studies of tottering (34) or leaner (32, 33), but may be caused by the complete removal of α1A as opposed to lessened efficiency of α1A function. Possible mechanisms for up- or down-regulation of non-P/Q-type Ca2+ channels include competition with α1A for ancillary subunits (2, 4, 49) or α1A-dependent changes in protein trafficking or gene expression (50). Excitatory synaptic transmission in α1A−/− hippocampal slices was spared despite the absence of P/Q-type channels, their contribution being offset, at least in part, by an increased reliance on both N- and R-type channels, as revealed by pharmacological dissection. This reinforces the idea that P/Q-, N-, and R-type channels all share the intrinsic capability of triggering fast neurotransmitter release at mammalian central synapses (47, 48).
The α1A−/− mice also gave fresh perspective on the relationship between Ca2+ channel function and motor behavior. The null mutants showed complete cessation of movement in brief episodes reminiscent of absence seizures in tottering or leaner (51), but also displayed a rolling form of ataxia (29) that progressively worsened up to the point of premature death, in contrast to tg and tgla. It will be interesting to learn whether the differences are accounted for by complete loss of P/Q-type channel function as opposed to its partial diminution in the spontaneous mutants (32–34). The α1A−/− animals provide a valuable experimental starting point for answering such questions and for approaching human diseases arising from defects in α1A, including certain forms of migraine (30, 52), epilepsy (51, 53), and ataxia (31).
Acknowledgments
We thank Y. Namkung, J. L. Kim, and D. Kim for advice in embryonic stem cell culture and embryo manipulation, J. R. Huguenard, R. J. Tolwani, and H. Bronte-Stewart for advice about neurological analysis, E. T. Kavalali and P. G. Mermelstein for advice about acute dissociation of Purkinje cells, M. P. Kong for animal care, and members of the Tsien laboratory for helpful discussion. This work is supported by the McCormick Foundation (E.S.P.-R.), a Howard Hughes Medical Institute Physician-Scientist Award (S.M.S.), a Howard Hughes Medical Institute predoctoral fellowship (D.B.W.), the Silvio Conte–National Institute of Mental Health Center for Neuroscience Research and the Mathers Charitable Trust (R.H.S. and R.W.T. and M.E.A.), the National Institute of Neurological Disorders and Stroke (R.W.T. and M.E.A.), and the Creative Research Initiative Program of the Ministry of Science and Technology, Korea (H.S.S.).
Abbreviations
- EPSP
excitatory postsynaptic potential
- wt
wild type
- 4-AP
4-aminopyridine
References
- 1.Mori Y, Friedrich T, Kim M S, Mikami A, Nakai J, Ruth P, Bosse E, Hofmann F, Flockerzi V, Furuichi T, et al. Nature (London) 1991;350:398–402. doi: 10.1038/350398a0. [DOI] [PubMed] [Google Scholar]
- 2.Dunlap K, Luebke J I, Turner T J. Trends Neurosci. 1995;18:89–98. [PubMed] [Google Scholar]
- 3.De Waard M, Gurnett C A, Campbell K P. In: Ion Channels. Narahashi T, editor. Vol. 4. New York: Plenum; 1996. pp. 41–87. [DOI] [PubMed] [Google Scholar]
- 4.Catterall W A. Cell Calcium. 1998;24:307–23. doi: 10.1016/s0143-4160(98)90055-0. [DOI] [PubMed] [Google Scholar]
- 5.Wheeler D B, Tsien R W. In: Calcium as a Cellular Regulator. Carafoli E, Lee C, editors. New York: Oxford Univ. Press; 1999. pp. 171–199. [Google Scholar]
- 6.Starr T V, Prystay W, Snutch T P. Proc Natl Acad Sci USA. 1991;88:5621–5625. doi: 10.1073/pnas.88.13.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J-F, Randall A D, Ellinor P T, Horne W A, Sather W A, Tanabe T, Schwarz T L, Tsien R W. Neuropharmacology. 1993;32:1075–1088. doi: 10.1016/0028-3908(93)90003-l. [DOI] [PubMed] [Google Scholar]
- 8.Randall A, Tsien R W. J Neurosci. 1995;15:2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tottene A, Moretti A, Pietrobon D. J Neurosci. 1996;16:6353–6363. doi: 10.1523/JNEUROSCI.16-20-06353.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludwig A, Flockerzi V, Hofmann F. J Neurosci. 1997;17:1339–1349. doi: 10.1523/JNEUROSCI.17-04-01339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Day N C, Wood S J, Ince P G, Volsen S G, Smith W, Slater C R, Shaw P J. J Neurosci. 1997;17:6226–6235. doi: 10.1523/JNEUROSCI.17-16-06226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westenbroek R E, Sakurai T, Elliott E M, Hell J W, Starr T V, Snutch T P, Catterall W A. J Neurosci. 1995;15:6403–6418. doi: 10.1523/JNEUROSCI.15-10-06403.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sather W A, Tanabe T, Zhang J-F, Mori Y, Adams M E, Tsien R W. Neuron. 1993;11:291–303. doi: 10.1016/0896-6273(93)90185-t. [DOI] [PubMed] [Google Scholar]
- 14.Stea A, Tomlinson W J, Soong T W, Bourinet E, Dubel S J, Vincent S R, Snutch T P. Proc Natl Acad Sci USA. 1994;91:10576–10580. doi: 10.1073/pnas.91.22.10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niidome T, Teramoto T, Murata Y, Tanaka I, Seto T, Sawada K, Mori Y, Katayama K. Biochem Biophys Res Commun. 1994;203:1821–1827. doi: 10.1006/bbrc.1994.2399. [DOI] [PubMed] [Google Scholar]
- 16.Llinás R M, Sugimori M, Hillman D E, Cherksey B. Trends Neurosci. 1992;15:351–355. doi: 10.1016/0166-2236(92)90053-b. [DOI] [PubMed] [Google Scholar]
- 17.Mintz I M, Adams M E, Bean B P. Neuron. 1992;9:85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- 18.Mintz I M, Venema V J, Swiderek K M, Lee T D, Bean B P, Adams M E. Nature (London) 1992;355:827–829. doi: 10.1038/355827a0. [DOI] [PubMed] [Google Scholar]
- 19.Scott V E, Felix R, Arikkath J, Campbell K P. J Neurosci. 1998;18:641–647. doi: 10.1523/JNEUROSCI.18-02-00641.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreno H, Rudy B, Llinas R. Proc Natl Acad Sci USA. 1997;94:14042–14047. doi: 10.1073/pnas.94.25.14042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mermelstein P G, Foehring R C, Tkatch T, Song W J, Baranauskas G, Surmeier D J. J Neurosci. 1999;19:7268–7277. doi: 10.1523/JNEUROSCI.19-17-07268.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bourinet E, Soong T W, Sutton K, Slaymaker S, Mathews E, Monteil A, Zamponi G W, Nargeot J, Snutch T P. Nat Neurosci. 1999;2:407–415. doi: 10.1038/8070. [DOI] [PubMed] [Google Scholar]
- 23.Beam K. Nat Neurosci. 1999;2:393–394. doi: 10.1038/8052. [DOI] [PubMed] [Google Scholar]
- 24.Moreno Davila H. In: Molecular and Functional Diversity of Ion Channels and Receptors. Rudy B, Seeburg P, editors. Vol. 868. New York: The New York Academy of Sciences; 1999. pp. 102–117. [DOI] [PubMed] [Google Scholar]
- 25.Gillard S E, Volsen S G, Smith W, Beattie R E, Bleakman D, Lodge D. Neuropharmacology. 1997;36:405–409. doi: 10.1016/s0028-3908(97)00046-4. [DOI] [PubMed] [Google Scholar]
- 26.Pinto A, Gillard S, Moss F, Whyte K, Brust P, Williams M, Stauderman K, Harpold M, Lang B, Newsom-Davis J, et al. Proc Natl Acad Sci USA. 1998;95:8328–8333. doi: 10.1073/pnas.95.14.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piedras-Rentería E S, Tsien R W. Proc Natl Acad Sci USA. 1998;95:7760–7765. doi: 10.1073/pnas.95.13.7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green M C, Sidman R L. J Hered. 1962;63:233–237. doi: 10.1093/oxfordjournals.jhered.a107180. [DOI] [PubMed] [Google Scholar]
- 29.Rhyu I J, Oda S, Uhm C S, Kim H, Suh Y S, Abbott L C. Neurosci Lett. 1999;266:49–52. doi: 10.1016/s0304-3940(99)00254-2. [DOI] [PubMed] [Google Scholar]
- 30.Ophoff R A, Terwindt G M, Vergouwe M N, van Eijk R, Oefner P J, Hoffman S M G, Lamerdin J E, Mohrenweiser H W, Bulman D E, Ferrari M, et al. Cell. 1996;87:543–552. doi: 10.1016/s0092-8674(00)81373-2. [DOI] [PubMed] [Google Scholar]
- 31.Zuchenko O, Bailey J, Bonnen P, Ashizawa T, Stockton D W, Amos C, Dobyns W B, Subramony S H, Zoghbi H, Lee C C. Nat Genet. 1997;15:62–69. doi: 10.1038/ng0197-62. [DOI] [PubMed] [Google Scholar]
- 32.Dove L S, Abbott L C, Griffith W H. J Neurosci. 1998;18:7687–7699. doi: 10.1523/JNEUROSCI.18-19-07687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lorenzon N M, Lutz C M, Frankel W N, Beam K G. J Neurosci. 1998;18:4482–4489. doi: 10.1523/JNEUROSCI.18-12-04482.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wakamori M, Yamazaki K, Matsunodaira H, Teramoto T, Tanaka I, Niidome T, Sawada K, Nishizawa Y, Sekiguchi N, Mori E, et al. J Biol Chem. 1998;273:34857–34867. doi: 10.1074/jbc.273.52.34857. [DOI] [PubMed] [Google Scholar]
- 35.Kraus R L, Sinnegger M J, Glossmann H, Hering S, Striessnig J. J Biol Chem. 1998;273:5586–5590. doi: 10.1074/jbc.273.10.5586. [DOI] [PubMed] [Google Scholar]
- 36.Hans M, Luvisetto S, Williams M E, Spagnolo M, Urrutia A, Tottene A, Brust P F, Johnson E C, Harpold M M, Stauderman K A, et al. J Neurosci. 1999;19:1610–1619. doi: 10.1523/JNEUROSCI.19-05-01610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jun K, Choi G, Yang S G, Choi K Y, Kim H, Chan G C, Storm D R, Albert C, Mayr G W, Lee C J, Shin H S. Learn Mem. 1998;5:317–30. [PMC free article] [PubMed] [Google Scholar]
- 38.Alger B E, Dhanjal S S, Dingledine R, Garthwaite J, Henderson G, King G L, Lipton P, North A, Schwartzkroin P A, Sears T A, et al. In: Brain Slices. Dingledine R, editor. New York: Plenum; 1984. pp. 381–437. [Google Scholar]
- 39.Uchitel O D, Protti D A, Sanchez V, Cherksey B D, Sugimori M, Llinas R. Proc Natl Acad Sci USA. 1992;89:3330–3333. doi: 10.1073/pnas.89.8.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hess E J, Wilson M C. Neuron. 1991;6:123–132. doi: 10.1016/0896-6273(91)90127-l. [DOI] [PubMed] [Google Scholar]
- 41.Olivera B M, Miljanich G P, Ramachandran J, Adams M E. Annu Rev Biochem. 1994;63:823–867. doi: 10.1146/annurev.bi.63.070194.004135. [DOI] [PubMed] [Google Scholar]
- 42.Adams M E, Mintz I M, Reily M D, Thanabal V, Bean B P. Mol Pharmacol. 1993;44:681–688. [PubMed] [Google Scholar]
- 43.Mintz I M, Sidach S. Soc Neurosci Abs. 1998;24:1021. [Google Scholar]
- 44.Campbell D B, Hess E J. Mol Pharmacol. 1999;55:23–31. doi: 10.1124/mol.55.1.23. [DOI] [PubMed] [Google Scholar]
- 45.Vogel S S, Smith R M, Baibakov B, Ikebuchi Y, Lambert N A. Proc Natl Acad Sci USA. 1999;96:5019–5024. doi: 10.1073/pnas.96.9.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wheeler D B, Randall A, Tsien R W. Science. 1994;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- 47.Wu L G, Borst J G, Sakmann B. Proc Natl Acad Sci USA. 1998;95:4720–4725. doi: 10.1073/pnas.95.8.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu L G, Westenbroek R E, Borst J G G, Catterall W A, Sakmann B. J Neurosci. 1999;19:726–736. doi: 10.1523/JNEUROSCI.19-02-00726.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brice N L, Dolphin A C. J Physiol (London) 1999;515:685–694. doi: 10.1111/j.1469-7793.1999.685ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sutton K G, McRory J, Guthrie H, Murphy T H, Snutch T P. Nature (London) 1999;401:800–804. doi: 10.1038/44586. [DOI] [PubMed] [Google Scholar]
- 51.Noebels J L, Sidman R L. Science. 1979;204:1334–1336. doi: 10.1126/science.572084. [DOI] [PubMed] [Google Scholar]
- 52.Miller R J. Trends Neurosci. 1997;20:189–192. doi: 10.1016/s0166-2236(96)01037-5. [DOI] [PubMed] [Google Scholar]
- 53.Fletcher C F, Lutz C M, O'Sullivan T N, Shaughnessy J D, Jr, Hawkes R, Frankel W N, Copeland N G, Jenkins N A. Cell. 1996;87:607–617. doi: 10.1016/s0092-8674(00)81381-1. [DOI] [PubMed] [Google Scholar]