Abstract
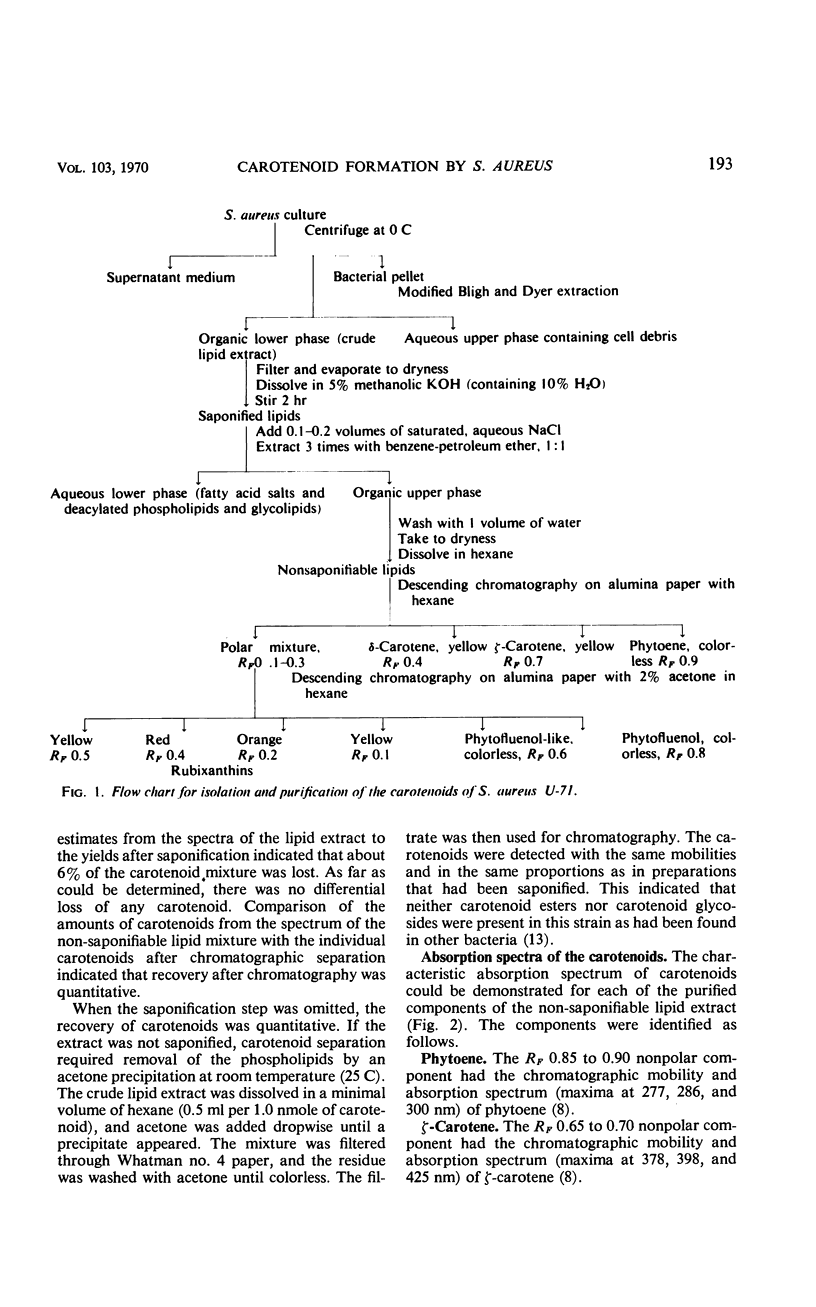
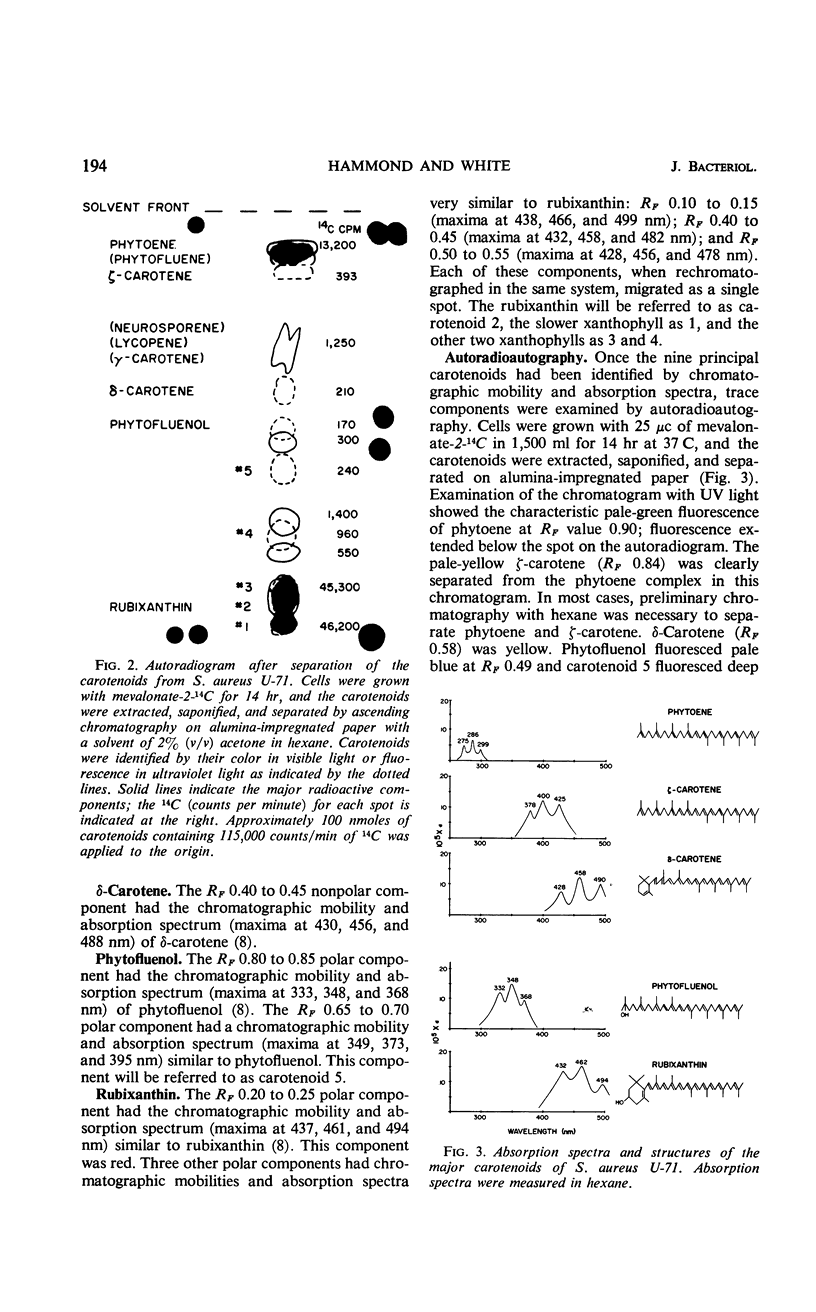
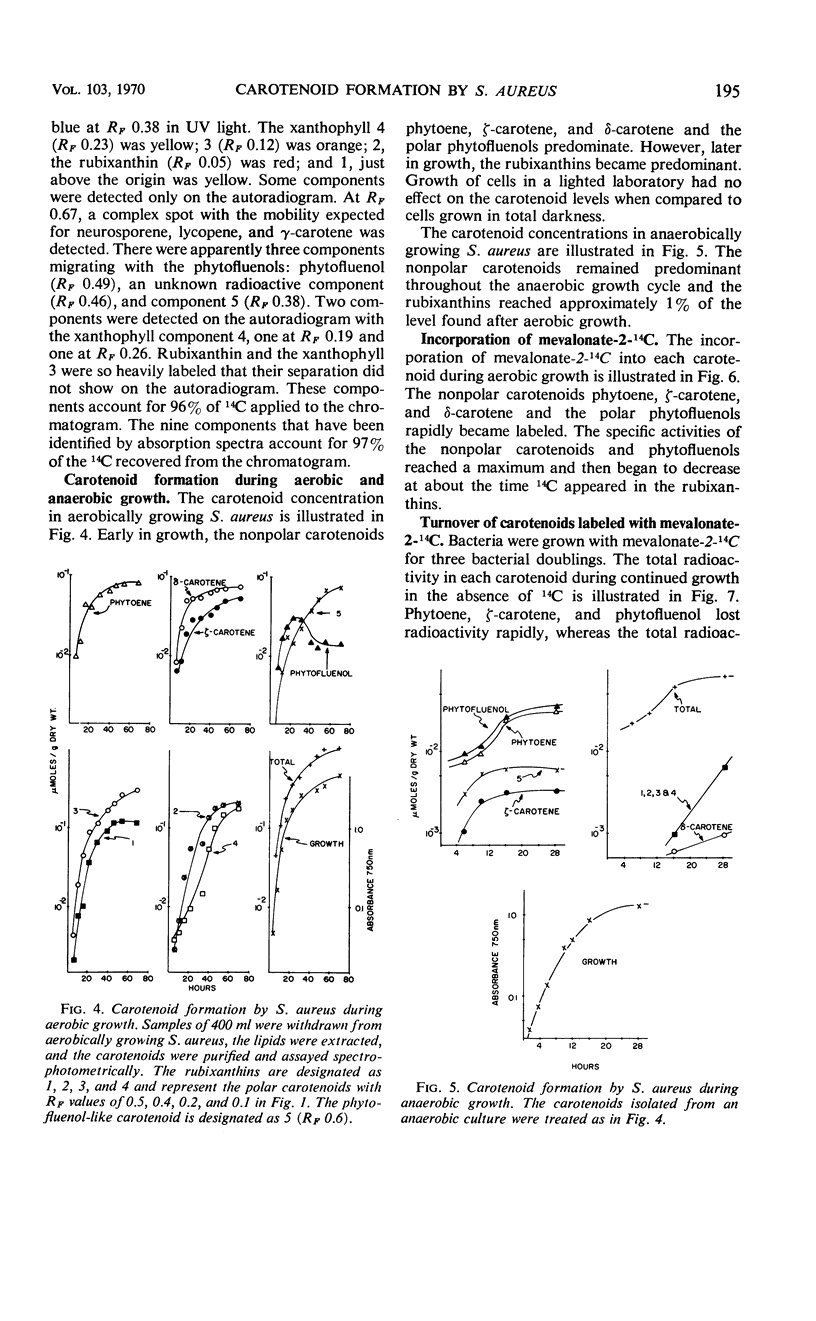
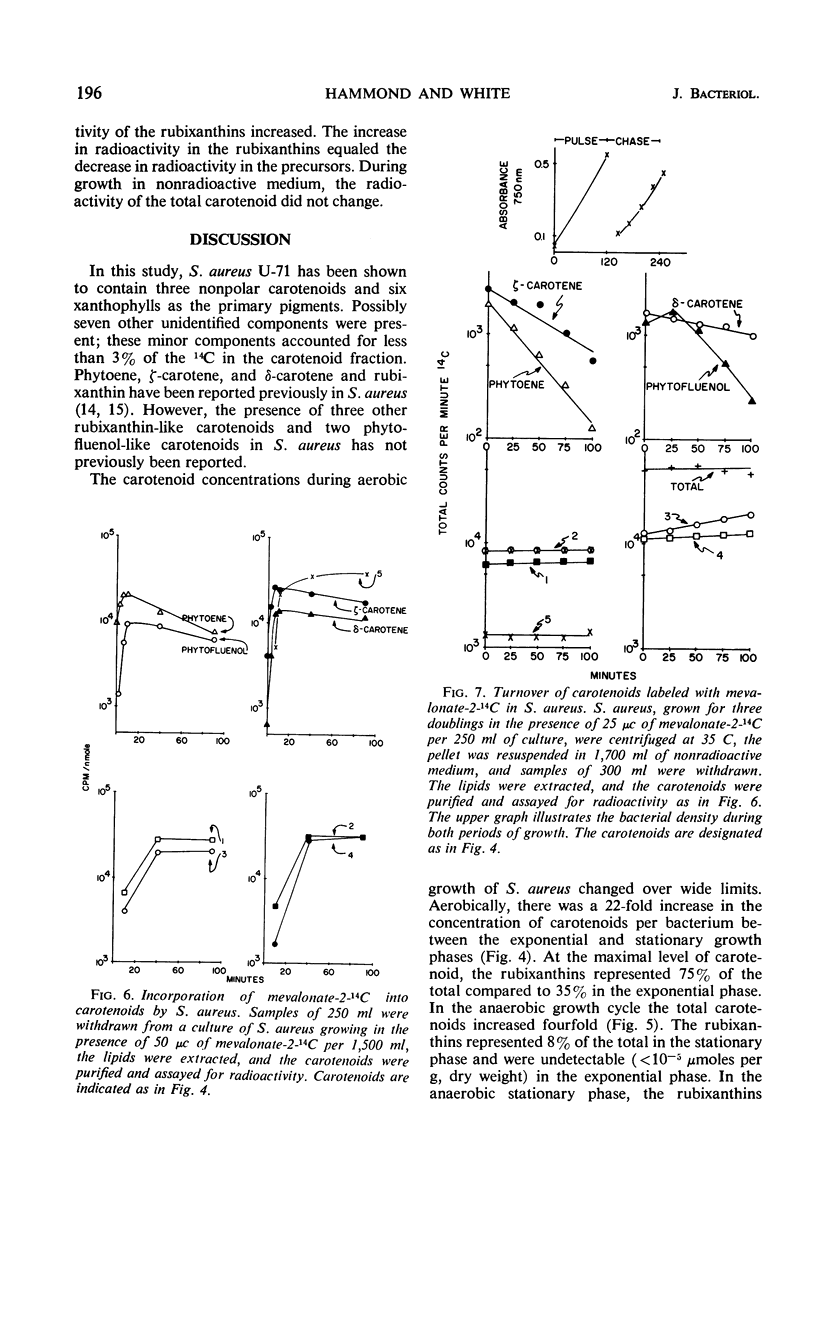
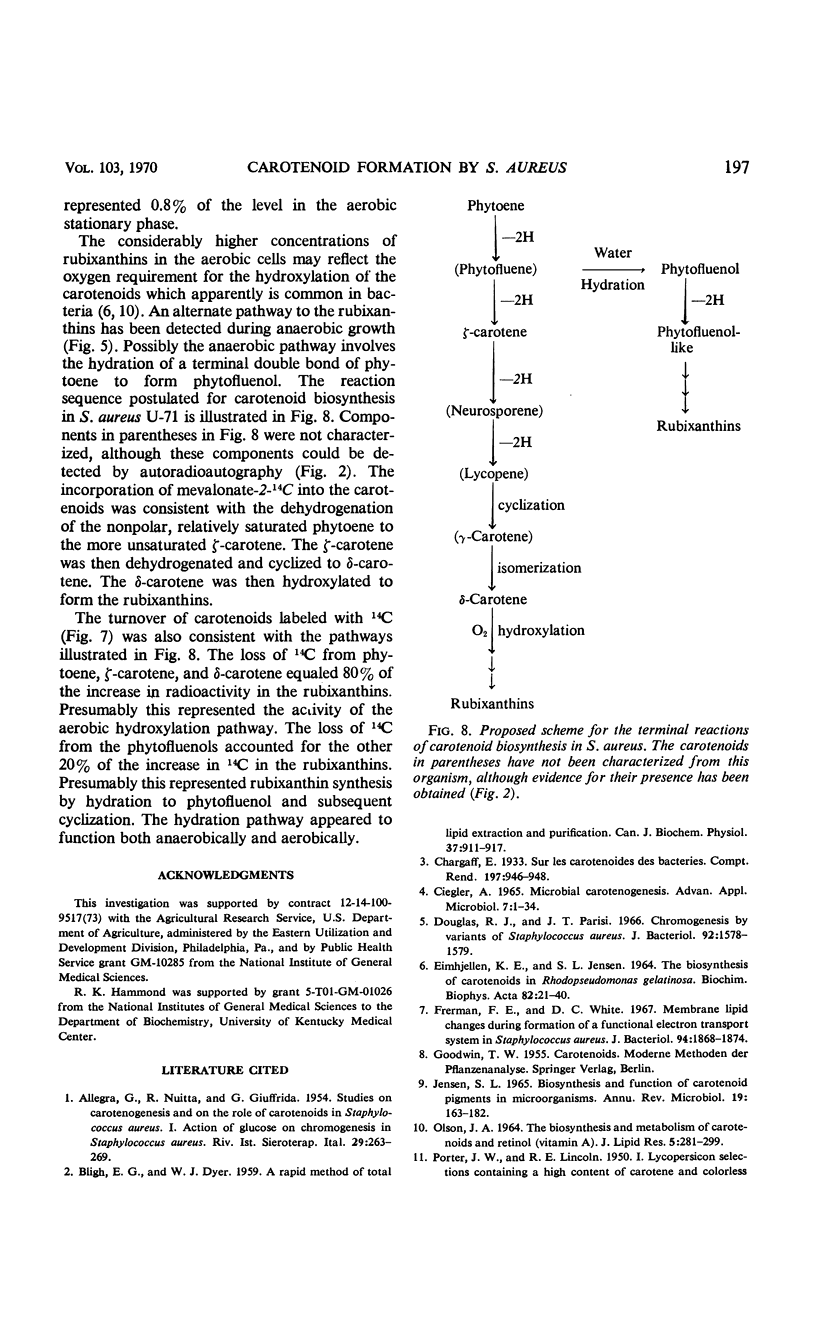
The carotenoid pigments of Staphylococcus aureus U-71 were identified as phytoene; ζ-carotene; δ-carotene; phytofluenol; a phytofluenol-like carotenoid, rubixanthin; and three rubixanthin-like carotenoids after extraction, saponification, chromatographic separation, and determination of their absorption spectra. There was no evidence of carotenoid esters or glycoside ethers in the extract before saponification. During the aerobic growth cycle the total carotenoids increased from 45 to 1,000 nmoles per g (dry weight), with the greatest increases in the polar, hydroxylated carotenoids. During the anaerobic growth cycle, the total carotenoids increased from 20 nmoles per g (dry weight) to 80 nmoles per g (dry weight), and only traces of the polar carotenoids were formed. Light had no effect on carotenoid synthesis. About 0.14% of the mevalonate-2-14C added to the culture was incorporated into the carotenoids during each bacterial doubling. The total carotenoids did not lose radioactivity when grown in the absence of 14C for 2.5 bacterial doublings. The total carotenoids did not lose radioactivity when grown in the absence of 14C for 2.5 bacterial doublings. The incorporation and turnover of 14C indicated the carotenes were sequentially desaturated and hydroxylated to form the polar carotenoids.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEGRA G., NIUTTA R., GIUFFRIDA G. Studi sulla carotenogenesi e sul ruolo dei carotenoidi nello Staphylococcus aureus. I. Azione del glucosio sulla cromogenesi dello Staphylococcus aureus (stipite Oxford). Riv Ist Sieroter Ital. 1954 May-Jun;29(3):263–269. [PubMed] [Google Scholar]
- Ciegler A. Microbial carotenogenesis. Adv Appl Microbiol. 1965;7:1–34. doi: 10.1016/s0065-2164(08)70382-4. [DOI] [PubMed] [Google Scholar]
- Douglas R. J., Parisi J. T. Chromogenesis by variants of Staphylococcus aureus. J Bacteriol. 1966 Nov;92(5):1578–1579. doi: 10.1128/jb.92.5.1578-1579.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerman F. E., White D. C. Membrane lipid changes during formation of a functional electron transport system in Staphylococcus aureus. J Bacteriol. 1967 Dec;94(6):1868–1874. doi: 10.1128/jb.94.6.1868-1874.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen S. L. Biosynthesis and function of carotenoid pigments in microorganisms. Annu Rev Microbiol. 1965;19:163–182. doi: 10.1146/annurev.mi.19.100165.001115. [DOI] [PubMed] [Google Scholar]
- Olson J. A. The biosynthesis and metabolism of carotenoids and retinol (vitamin A). J Lipid Res. 1964 Jul;5(3):281–299. [PubMed] [Google Scholar]
- RILLING H. C. Anaerobic carotenoid synthesis by an aerobic microorganism. Biochim Biophys Acta. 1962 Nov 19;65:156–158. doi: 10.1016/0006-3002(62)90164-6. [DOI] [PubMed] [Google Scholar]
- SMITH P. F. THE CAROTENOID PIGMENTS OF MYCOPLASMA. J Gen Microbiol. 1963 Sep;32:307–319. doi: 10.1099/00221287-32-3-307. [DOI] [PubMed] [Google Scholar]
- Sobin B., Stahly G. L. The Isolation and Absorption Spectrum Maxima of Bacterial Carotenoid Pigments. J Bacteriol. 1942 Sep;44(3):265–276. doi: 10.1128/jb.44.3.265-276.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C. Lipid composition of the electron transport membrane of Haemophilus parainfluenzae. J Bacteriol. 1968 Oct;96(4):1159–1170. doi: 10.1128/jb.96.4.1159-1170.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


