Abstract
Ischemia bowel remains a critical problem resulting in up to 80% mortality. The loss of gut barrier function plays an important role. Our previous studies have shown that administration of adrenomedullin (AM), a novel vasoactive peptide, and its binding protein (AMBP-1), reduces the systemic inflammatory response and organ injury after systemic ischemia induced by hemorrhagic shock. However, it remains unknown whether administration of AM/AMBP-1 preserves gut barrier function after gut ischemia reperfusion (I/R). We therefore hypothesized that AM/AMBP-1 prevents structural and functional damages to the intestinal mucosa after gut I/R. To test this, gut ischemia was induced by placing a microvascular clip across the superior mesenteric artery (SMA) for 90 min in male adult rats. After release of the SMA clamp, AM (12 μg/kg BW) and AMBP-1 (40 μg/kg BW) in combination or vehicle (1-ml normal saline) were administered intravenously over a period of 30 min. The mucosal barrier function in the small intestine was assessed in an isolated everted ileum sac using fluorescein-isothiocyanate dextran (FD4) at 4 h after AM/AMBP-1 treatment. FD4 clearance was used as a measure of gut permeability. In additional groups of animals, blood and small intestine samples were collected at 4 h after the treatment. Morphological changes in the small intestine were assessed by H-E staining. Serum concentrations of alanine aminotransferase, aspartate aminotransferase, total bilirubin, direct bilirubin, lactate and lactate dehydrogenase were also assessed. The gene expression and protein levels of TNF-α in the small intestine were determined by RT-PCR and ELISA, respectively. Our results showed that administration of AM/AMBP-1 significantly attenuated the development of intestinal mucosal hyperpermeability during the reperfusion. Treatment with AM/AMBP-1 dramatically improved I/R-induced intestinal mucosal damages, attenuated remote organ injury, and downregulated gene expression and protein levels of TNF-α in the small intestine. In conclusion, AM/AMBP-1 attenuates structural and functional damages to the intestinal mucosa, and it appears to be a novel treatment for reperfusion injury after gut ischemia. The beneficial effect of AM/AMBP-1 on gut barrier function after I/R is associated with downregulation of TNF-α.
Keywords: Ischemia-reperfusion, intestines, permeability, tissue injury, TNF-α
Introduction
Gut injury as a result of ischemia and subsequent reperfusion (I/R) is a life-threatening clinical emergency. Interruption of blood supply causes ischemia, which rapidly damages metabolically active tissues. Restoration of blood flow after a period of intestinal ischemia is necessary to maintain cell function and viability. However, restoration of blood flow to the ischemic tissues can initiate a cascade of pathophysiologic responses that lead to additional cell or tissue injury. Loss of the barrier function of the gastrointestinal tract has been implicated as a potential source of multiple organ failure under such conditions. Even though numerous modalities and substances have been studied to reduce I/R-induced organ injury, the mortality after acute mesenteric ischemia remains high [1, 2]. It is obvious that there is an urgent need for the development of an effective novel approach for the treatment of patients with gut I/R.
Adrenomedullin (AM) is a potent vasodilatory peptide that was originally isolated from human pheochromocytoma [3]. Infusion of AM causes vasodilatation, diuresis, and natriuresis and inhibits aldosterone secretion in normal animals [4]. In addition to its well-known vasodilatory effects, AM also modulates production of inflammatory cytokines. Kamoi et al reported that AM inhibited secretion of cytokine-induced neutrophil chemoattractant in rat alveolar macrophages stimulated with endotoxin [5]. Furthermore, AM suppresses apoptosis of rat endothelial cells via a cAMP-independent mechanism [6]. However, the regulation of AM activity under various conditions remained largely unknown until the discovery of its novel specific binding protein, AM binding protein-1 (AMBP-1). AMBP-1, a 120-and/or 140-kDa protein complex, has been purified from human plasma and is identical to human complement factor H [7]. Complement factor H inhibits the alternative complement pathway [8], binds to cell surfaces, and modulates neutrophil and monocyte function [9, 10]. The discovery of the interaction between AM and AMBP-1 has opened a new avenue for further understanding of the AM biology. Our recent studies demonstrated that AMBP-1 synergistically enhanced AM-induced vascular relaxation, and administration of AM and AMBP-1 in combination significantly reduces proinflammatory cytokines and improves organ function in rat models of polymicrobial sepsis and hemorrhagic shock [11, 12]. However, it remains unknown whether administration of AM/AMBP-1 preserves gut barrier function after gut I/R. This study was conducted to test the hypothesis that AM/AMBP-1 prevents structural and functional damages to the intestinal mucosa after gut I/R.
Materials and Methods
Experimental Animals
Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA), weighing 270–350 g, were used in this study. The animals were housed in a temperature-controlled room on a 12-h light/dark cycle and fed on a standard Purina rat chow diet. All surgical procedures were performed using aseptic technique. All procedures described here were performed in accordance with the National Institutes of Health guidelines for the use of experimental animals. This project was approved by the Institutional Animal Care and Use Committee of the Feinstein Institute for Medical Research.
Gut Ischemia-Reperfusion Model
Prior to the induction of ischemia, rats were fasted overnight but allowed water ad libitum. Rats were anesthetized with isoflurane inhalation and the ventral neck, abdomen and groin were shaved and washed with 10% povidone iodine. A catheter (PE-50 tubing) was placed in the femoral vein after carefully separating the femoral nerve and blood vessels. A 2-cm midline abdominal incision was performed. A microvascular clip was placed across the superior mesenteric artery (SMA) for 90 min. The microvascular clip was then removed to allow reperfusion, and the animals were randomly assigned to various groups. Sham animals (i.e., control animals) underwent the same surgical procedure with the exception of the SMA clamping. The animals were under anesthesia from the beginning of the procedure to the end of resuscitation. They were awakened after the completion of treatment.
Administration of AM/AMBP-1
Immediately after removing the microvascular clip, AM (12 μg/kg body weight, Phoenix Pharmaceuticals, Belmont, CA) and AMBP-1 (40 μg/kg body weight, Cortex Biochem, San Leandro, CA) in combination or vehicle (1 ml normal saline) was administered intravenously over a period of 30 min using a Harvard infusion pump (Harvard Apparatus, Holliston, MA) via a femoral vein catheter. The purity of synthetic rat AM is more than 99% (by HPLC). Human AMBP-1 is isolated from human serum/plasma with a purity of more than 98% (by SDS-PAGE). The human AMBP-1 preparation has been tested at the serum/plasma donor level by FDA-approved methods and found negative for HIV I/II, and HCV antibodies and hepatitis B surface antigens. Animals were then anesthetized at 4 h after the completion of treatment (i.e., 4.5 h after reperfusion) or sham operation for collection of blood and tissue samples. Blood samples were centrifuged at 3000 g for 10 min at 4°C, and the serum samples were stored at −80°C until assayed. The doses of AM and AMBP-1 were selected based on our previous experiences in sepsis and hemorrhage [13, 14].
Determination of Gut Permeability
Small intestinal mucosal barrier function was assessed by using the ex-vivo isolated everted ileum sac as originally described by Wattanasirichaigoon et al in 1999 [15] and used by others recently [16–18]. Briefly, at 4 h reperfusion or sham operation, everted gut sacs were prepared in ice-cold modified Krebs-Henseleit bicarbonate buffer (KHBB, pH 7.4). Fluorescein-isothiocyanate dextran with a molecular weight of 4000 Da (FD4), was used as a permeability probe. One end of the ileal segment was closed with a 3–0 silk ligature. The gut sac was everted onto a thin rod, then connected to a 3-ml plastic syringe containing KHBB, and secured with a 3–0 silk ligature. The everted gut sacs were gently distended by injecting 1.5 mL of KHBB in a 250ml-beaker containing 80 mL of KHBB with added FD4 (20 μg/mL). The beakers were incubated for 30 min in a shaker bath at a frequency of 80–100 shakes/min. The incubation medium in the beaker was temperature jacketed at 37°C, and was continuously bubbled with a gas mixture containing 95% O2 and 5% CO2. A 1.0-ml sample was taken from the beaker at the beginning of the incubation to determine the initial FD4 concentration of the mucosal side. After a 30 min of incubation time, the fluid was aspirated from the inside of the sac to determine FD4 concentration of the serosal side. The length and the diameter of the gut sac were then measured. The serosal and mucosal samples were cleared by centrifugation for 10 min at 1000 g at 4°C. Fluorescence measurements were made with a CYTOFLUOR 2 fluorescence plate reader (PerSeptive Biosystems, Framingham, MA) at an excitation wavelength of 480 nm and an emission wavelength of 520 nm. Gut permeability was expressed as the mucosal-to-serosal clearance of FD4 as described in detail by Wattanasirichaigoon et al [15].
Examination of Gut Histopathology
The morphological alterations in the gut were examined by light microscopy. Briefly, gut samples were obtained at 4 h after reperfusion or sham operation and submerged in 10% formalin in neutral buffered solution (Sigma, St. Louis, MO) for immediate fixation and later embedded in paraffin. The tissue blocks were then sectioned at a thickness of 5 μm, floated on warm water, and transferred to glass slides, where they were stained with hematoxylin and eosin (H-E), dehydrated, and coverslipped. The scoring system devised by Chiu et al [19] was used to quantify the histological appearance (this was performed by an observer blinded to the treatment the animal received). The scoring system was as follows:
| Grade | Description |
|---|---|
| 0 | Normal villi |
| 1 | Subepithelial space top of villus |
| 2 | Extension of subepithelial space at top of villus with moderate separation of mucosa from lamina propria |
| 3 | Extensive subepithelial separation from the lamina propria down the sides of the villi, ulceration at the villous tips |
| 4 | Denuded villi |
| 5 | Disintegration of lamina propria |
Determination of Circulating Transaminases, Bilirubin, Lactate and Lactate Dehydrogenase (LDH)
Serum concentrations of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (T-Bil), direct bilirubin (D-Bil), lactate and LDH were determined by using assay kits according to the manufacturer's instructions (Pointe Scientific, Lincoln Park, MI).
Determination of Tissue TNF-α Gene Expression
Determination of TNF-α gene expression was performed by reverse transcription polymerase chain reaction (RT-PCR). Total cellular RNA was extracted from small intestine tissues using TRIzol Reagent (Invitrogen, Carlsbad, CA). Briefly, 150 mg of tissue was homogenized in 1.5 mL of TRIzol Reagent, and the homogenate was separated into aqueous phase and organic phase by 1-bromo-3-chloropropane addition and centrifugation. RNA was precipitated from the aqueous phase by addition of isopropanol and washed with ethanol. The pellet was dissolved in RNA secure reagent (Ambion, Austin, TX). The concentration and purity of the isolated RNA were quantitated using spectrophotometry by measuring absorbance at 260 and 280 nm. Eight μg RNA from each of the samples were reverse-transcribed in a 40 μl reaction volume, containing 5 mM MgCl2, 0.25 mM dNTP Mix, 2.5 μM oligo d(T)12-18 primer, 40 U of RNase inhibitor, and 100 U of reverse transcriptase with PCR buffer (Invitrogen). The reverse transcription reaction samples were incubated at 42°C for 1 h and followed by incubation at 95°C for 5 min. The resulting complementary DNAs were amplified by polymerase chain reaction. Rat glyceraldehyde 3-phosphate dehydrogenase (G3PDH) served as a housekeeping gene. The primer of G3PDH and TNF-α are as follows: G3PDH (983 bp; M17701), 5′-TGA AGG TCG GTG TCA ACG GAT TTG GC-3′ (forward), 5′-CAT GTA GGC CAT GAG GTG CAC CAC-3′ (reverse); TNF-α (694 bp; L00981), 5′-CCC AGA CCC TCA CAC TCA GA-3′ (forward), 5′-GCC ACT ACT TCA GCA TCT CG-3′ (reverse). PCR was carried out at 28 cycles for G3PDH and 30 cycles for TNF-α in 25 μl reaction volume, containing 2 mM of MgCl2, 0.2 mM dNTP Mix, and 2 U of Amplitaq DNA polymerase with PCR buffer. The amplification cycle for G3PDH consisted of denaturation at 94°C for 45 sec, annealing at 60°C for 45 sec, and extension at 72°C for 2 min. The amplification cycle for TNF-α consisted of denaturation at 94°C for 45 sec, annealing at 60°C for 45 sec, and extension at 72°C for 90 sec. Each cycle followed by a final extension for 10 min at 72°C. PCR was conducted in a Bio-Rad thermal cycler. Following the procedures of RT-PCR, the reaction products were electrophoresed in 1.6% TBE-agarose gel containing 0.22 μg/mL ethidium bromide. The gel was photographed on Polaroid film. The band density was determined by a digital image analyzer (Bio-Rad, Hercules, CA).
Determination of Tissue Levels of TNF-α
Rat small intestine (0.1 g) was homogenized in 1 mL of lysis buffer (10 mM triethanolamine-buffered saline, 1 mM EDTA, 1 mM EGTA, 2 mM Na orthovanadate, 0.2mM phenyl-methylsulfonyl fluoride, 2 μg/mL leupeptin, 2 μg/mL aprotinin, 1% Triton-X 100), and cleared by centrifugation at 14,000 rpm for 15 min at 4°C. TNF-α levels were quantified using enzyme-linked immunosorbent assay (ELISA) kits, specifically for TNF-α (BD Biosciences, San Diego, CA). The assay was carried out according to the instructions provided by the manufacturer. TNF-α levels were normalized to the protein concentration in the sample.
Statistical Analysis
All data are expressed as means ± SE and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls test. Differences in values were considered significant if P<0.05.
Results
Alterations in Gut Permeability after Ischemia Reperfusion
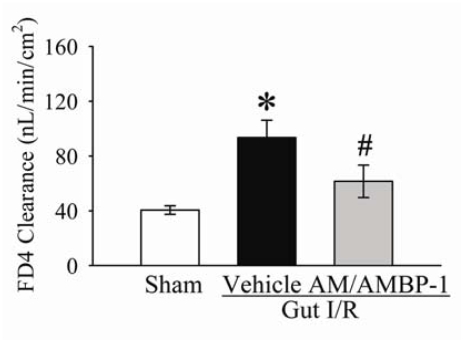
As indicated in Figure 1, gut permeability increased by 145% at 4 h after gut I/R and vehicle administration (normal saline) (P<0.05). Treatment of AM/AM BP-1 decreased gut permeability by 44% (P<0.05), and it was not statistically different from the sham-operated animals.
Figure 1.
Alterations in intestinal mucosal permeability to fluorescein isothiocyanate dextran with a molecular weight of 4000 Da (FD4) in sham-operated animals (Sham) and ischemia-reperfusion animals treated with normal saline (Vehicle) or AM/AMBP-1 at 4 h after the completion of treatment. Data are presented as means ± SE (n=6), and compared by one-way ANOVA and Student-Newman-Keuls test: * P<0.05 versus Sham group; # P<0.05 versus vehicle group.
Alterations in Morphology of the Small Intestine
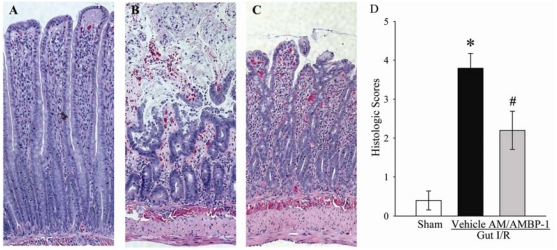
In terms of the histopathological changes, severe intestinal mucosal damage with areas of denudation of villi was observed microscopically in the rat intestine after I/R (Figure 2B) as compared with sham-operated controls (Figure 2A). Although mucosal damages were apparent as evidenced by reduction of villus height and some hemorrhage in the treatment group with AM/AMBP-1, mucosal structure was obviously preserved compared with vehicle-treated animals (Figure 2C). As indicated in Figure 2D, the gut injury score was significantly increased at 4 h after gut I/R and vehicle treatment as compared with that in sham operated animals (P<0.05). Administration of AM/AMBP-1 after gut ischemia markedly reduced the gut injury score by 42% (P<0.05).
Figure 2.
Alterations in morphology of the small intestine. A. Photomicrograph of a small intestinal section from a sham-operated rat. B. An I/R rat at 4 h after gut ischemia-reperfusion treated with vehicle. C. An I/R rat at 4 h after gut ischemia-reperfusion treated with AM/AMBP-1. D. Histopathological scoring of gut injury in sham-operated animals (Sham) and ischemia-reperfusion animals treated with normal saline (Vehicle) or AM/AMBP-1 at 4 h after the completion of treatment. Data are presented as means ± SE (n=5) and compared by one-way ANOVA and Student-Newman-Keuls method. * P<0.05 versus Sham group; # P<0.05 versus vehicle group. Original magnification × 200.
Alterations of Serum AST, ALT, T-Bil, D-Bil, Lactate and LDH Levels
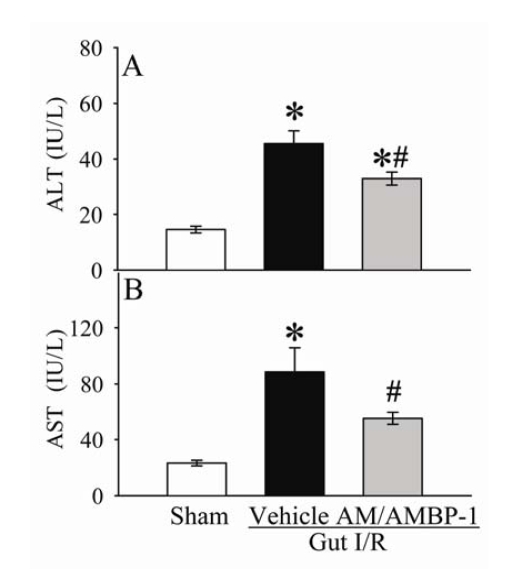
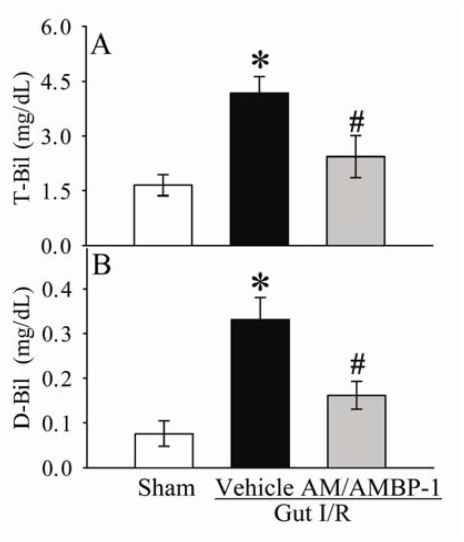
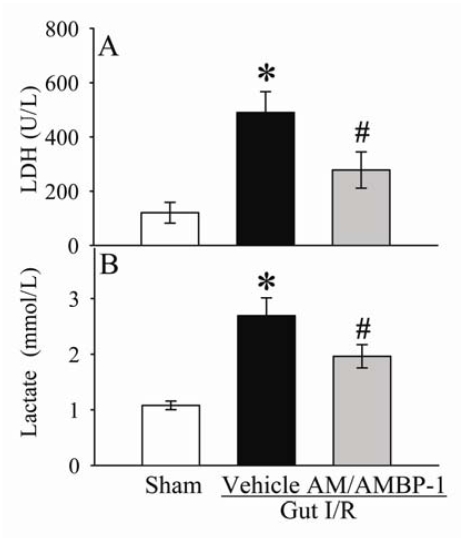
As shown in Figures 3A-B, serum levels of ALT and AST increased by 207% and 283% (P<0.05), respectively, at 4 h after gut I/R and vehicle administration. Administration of AM/AMBP-1 reduced serum levels of ALT and AST by 28% and 37% (P<0.05), respectively. Similarly, circulating levels of T-Bil and D-Bil in the vehicle group were significantly elevated by 153% and 334% (P<0.05, Figures 4A-B), respectively, as compared with sham-operated animals. AM/AMBP-1 treatment decreased serum T-Bil levels by 42% (P<0.05) and D-Bil levels by 51% (P<0.05). Compared with sham operated animals, serum levels of LDH increased by 307% at 4 h after gut I/R and vehicle administration (P<0.05, Figure 5A). AM/AMBP-1 treatment significantly reduced serum levels of lactate by 43% (P<0.05).
Figure 3.
Alterations in serum levels of alanine aminotransferase (ALT) (A) and aspartate aminotransferase (AST) (B) in sham-operated animals (Sham) and ischemia-reperfusion animals treated with normal saline (Vehicle) or AM/AMBP-1 at 4 h after the completion of treatment. Data are presented as mean ± SE (n=6), and compared by one-way ANOVA and Student-Newman-Keuls test. * P<0.05 versus sham group; # P<0.05 versus vehicle group.
Figure 4.
Alterations in serum levels of total bilirubin (T-Bil) (A) and direct bilirubin (D-Bil) (B) in sham-operated animals (Sham) and ischemia-reperfusion animals treated with normal saline (Vehicle) or AM/AMBP-1 at 4 h after the completion of treatment. Data are presented as mean ± SE (n=5-6), and compared by one-way ANOVA and Student-Newman-Keuls test. * P<0.05 versus sham group; # P<0.05 versus vehicle group.
Figure 5.
Alterations in serum levels of lactate dehydrogenase (LDH) (A) and lactate (B) in sham-operated animals (Sham) and ischemia-reperfusion animals treated with normal saline (Vehicle) or AM/AMBP-1 at 4 h after the completion of treatment. Data are presented as mean ± SE (n=6-7), and compared by one-way ANOVA and Student-Newman-Keuls test. * P<0.05 versus sham group; # P<0.05 versus vehicle group.
Similarly, serum levels of lactate increased by 149% at 4 h after gut I/R and vehicle administration (P<0.05, Figure 5B). AM/AMBP-1 treatment significantly reduced serum levels of lactate by 37% (P<0.05, Figure 5B).
Alterations of Gene Expression and Protein Level of TNF-α in the Small Intestine
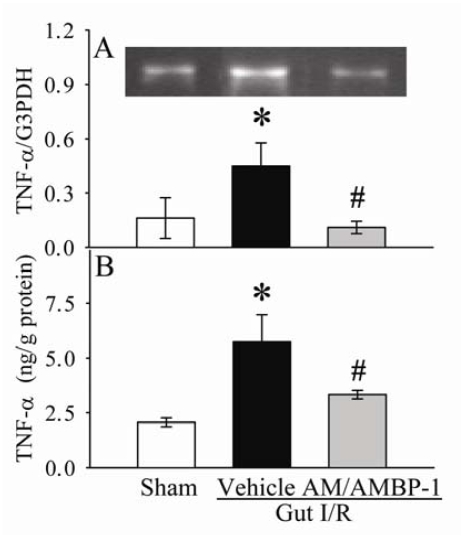
The gene expression and protein level of TNF-α in the small intestine was assessed by RT-PCR and ELISA, respectively. As demonstrated in Figures 6A-B, TNF-α gene expression and protein level were significantly upregulated by 181% and 171% (P<0.05), respectively, in I/R vehicle group as compared with sham-operated animals. With AM/AMBP-1 treatment, I/R-induced upregulation of TNF-α expression and protein level was significantly attenuated by 77% and 42% (P<0.05), respectively.
Figure 6.
Alterations in mRNA expression of TNF-α (A) and protein levels of TNF-α (B) in the small intestine of sham-operated animals (Sham) and ischemia-reperfusion animals treated with normal saline (Vehicle) or AM/AMBP-1 at 4 h after the completion of treatment. Data are presented as mean ± SE (n=6), and compared by one-way ANOVA and Student-Newman-Keuls test. * P<0.05 versus Sham group; # P<0.05 versus Vehicle group.
Discussion
Our recent studies have shown that AM/AMBP-1 administration decreases lung edema and reduces neutrophil infiltration in the lungs after gut I/R [20]. The current study confirms the previously documented finding that ischemia-reperfusion injury to the intestine results in an increase in intestinal permeability in vivo and leads to distant organ injury [21, 22]. The data validates the hypothesis that the combination of AM and its binding protein AMBP-1 preserved gut barrier function and improved organ function under such conditions.
Superior mesenteric artery occlusion is a widely used model to induce gut ischemia in rodents. Like most of animal studies, variations present within the group. However, our result showed that the variations within the group are much less than the differences among groups. Moreover, the absence of collateral arteries is uncommon in patients with acute mesenteric ischemia. Therefore, it appears that ligation of the duodenal collateral arteries may not be clinically relevant. The clinical features of gut ischemia originate from local and systemic responses. Organ injury induced by I/R is not necessarily limited to the ischemic organ, but also involve distant non-ischemic organs. It is now widely accepted that local and remote organ injury following intestinal I/R is the result of the actions of an integrated network of soluble inflammatory mediators and a variety of inflammatory cells. The loss of gut barrier function plays an important role in initiating this process. The gastrointestinal tract not only functions as a site for nutrient absorption but also acts as a barrier between the circulation and noxious substances such as intraluminal organisms entering the circulation [23]. Maintenance of normal epithelial structure and function is important in preventing transcellular and paracellular movement of large molecules and bacteria [24]. Gut I/R injury is one of the most common causes of gut barrier disruption seen in surgical practice [25]. The gut mucosa suffers most, as it depends on a high oxygen demand even in the steady state. The architecture of the mucosal microvasculature exposes the tip of the villus at the highest risk for ischemia in low flow states. The epithelial integrity is compromised and as such, the intestinal barrier dysfunction ultimately allows translocation of endotoxin and whole bacterial organisms [25]. Activation of leukocytes and stimulation of cytokine synthesis cause a sustained inflammatory response syndrome following reperfusion [26]. Increased intestinal permeability has been reported to be associated with an increased risk of complications, multiply organ failure, or even mortality in critically ill patients [27–29]. In the present study, we have demonstrated that administration of AM in combination with AMBP-1 provided significant protection against the development of intestinal mucosal hyperpermeability to the fluorescent macromolecule, FD4, as compared to that measured in gut I/R vehicle treated rats. Furthermore, improved gut barrier function after AM/AM BP-1 treatment is associated with reduced remote organ injury as indicated by marked decreases in significantly elevated serum concentrations of AST, ALT, total and direct bilirubin, LDH and lactate after gut I/R. Reperfusion injury after gut ischemia can induce a critical form of circulatory collapse characterized by severe hypotension, decreased cardiac output, and impaired organ blood flow. All these will contribute to the lactate elevation. The result that AM/AMBP-1 decreased lactate levels indicates that AM/AMBP-1 improves perfusion both locally and systemically. Our previous studies have demonstrated that only a combination of AM and AMBP-1 but not each agent alone is required for the treatment of global ischemia/reperfusion elicited by hemorrhagic shock and resuscitation [12, 14, 30]. Since AMBP-1 enhances the biological effects of AM under normal and pathophysiological conditions [31], we believed that a combination of AM and AMBP-1 is also required for the treatment of gut I/R.
Multiple mechanisms are involved in gut barrier dysfunction after ischemia reperfusion. Among them, inflammatory responses play an important role. Proinflammatory cytokines, such as TNF-α, interferon-γ, IL-1β have been shown to increase the permeability of enterocyte-like monolayers growing on filters in diffusion chambers [32–35]. Recombinant TNF-α increases apoptosis and permeability of intestinal epithelial cell under shock condition of bacterial exposure or hypoxia-reoxygenation [36]. Moreover, TNF-α increases intestinal tight junction permeability which is mediated by an increase in myosin light chain kinase [37]. In this regard, various anti-inflammatory pharmacological strategies also have been shown to be effective. For example, Luyer et al recently reported that treatment of rats, with a high-fat enteral diet, protects against late bacterial translocation after hemorrhagic shock, probably by downregulating the inflammatory response [38]. In the present study, we focus on the effect of AM/AM BP-1 on gut injury. Our result shows that treatment with AM/AM BP-1 downregulated gene expression and protein levels of TNF-α in the small intestine, which is evaluated to reflect the proinflammatory response. The downregulatory effect of AM/AMBP-1 on proinflammatory cytokines after gut I/R appears to be due to the direct action since our previous studies indicate that AM and AMBP-1 together effectively suppress endotoxin-induced TNF-α expression and release from RAW264.7 cells (a murine macrophage cell line) as well as Kupffer cells isolated from normal rats [39]. Although the precise cause and effect relationship between downregulation of TNF-α and the beneficial effect of AM/AMBP-1 cannot be established in this study, decreased levels of TNF-α have been shown to reduce the organ injury under various pathological situations [11]. Goldman et al reported that treatment with an anti-TNF antibody ameliorates bacterial translocation in rats after systemic ischemia induced by hemorrhagic shock [40]. Therefore, we believe that AM/AMBP-1's beneficial effect on gut barrier function after I/R is mainly mediated through downregulation of proinflammatory cytokines.
In summary, our results indicate that gut permeability significantly increases after 90 min ischemia followed by 4 h reperfusion. Gut I/R induces remote organ injury as evidenced by increased serum levels of ALT, AST and bilirubin. Administration of AM/AMBP-1 after gut I/R dramatically improves gut hyperpermeability and attenuates organ injury. These beneficial effects of AM/AMBP-1 after gut I/R are associated with downregulation of intestinal TNF-α. Thus, AM/AMBP-1 should be considered as a novel approach to prevent reperfusion injury after gut ischemia.
Acknowledgments
This study was supported in part by National Institutes of Health grant R01 HL076179 and R01GM057468 (PW).
References
- 1.Oldenburg WA, Lau LL, Rodenberg TJ, Edmonds HJ, Burger CD. Acute mesenteric ischemia: a clinical review. Arch Int Med. 2004;164:1054–1062. doi: 10.1001/archinte.164.10.1054. [DOI] [PubMed] [Google Scholar]
- 2.Berlanga J, Prats P, Remirez D, Gonzalez R, Lopez-Saura P, Aguiar J, Ojeda M, Boyle JJ, Fitzgerald AJ, Playford RJ. Prophylactic use of epidermal growth factor reduces ischemia/reperfusion intestinal damage. Am J Pathol. 2002;161:373–379. doi: 10.1016/S0002-9440(10)64192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, Eto T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192:553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- 4.Samson WK. Adrenomedullin and the control of fluid and electrolyte homeostasis. Annu Rev Physiol. 1999;61:363–389. doi: 10.1146/annurev.physiol.61.1.363. [DOI] [PubMed] [Google Scholar]
- 5.Kamoi H, Kanazawa H, Hirata K, Kurihara N, Yano Y, Otani S. Adrenomedullin inhibits the secretion of cytokine-induced neutrophil chemoattractant, a member of the interleukin-8 family, from rat alveolar macrophages. Biochem Biophys Res Commun. 1995;211:1031–1035. doi: 10.1006/bbrc.1995.1914. [DOI] [PubMed] [Google Scholar]
- 6.Kato H, Shichiri M, Marumo F, Hirata Y. Adrenomedullin as an autocrine/paracrine apoptosis survival factor for rat endothelial cells. Endocrinology. 1997;138:2615–2620. doi: 10.1210/endo.138.6.5197. [DOI] [PubMed] [Google Scholar]
- 7.Pio R, Martinez A, Unsworth EJ, Kowalak JA, Bengoechea JA, Zipfel PF, Elsasser TH, Cuttitta F. Complement factor H is a serum binding protein for adrenomedullin. The resulting complex modulates the bioactivities of both partners. J Biol Chem. 2001;276:12292–12300. doi: 10.1074/jbc.M007822200. [DOI] [PubMed] [Google Scholar]
- 8.Harrison RA, Lachmann PJ. The physiological breakdown of the third component of human complement. Mol Immunol. 1980;17:9–20. doi: 10.1016/0161-5890(80)90119-4. [DOI] [PubMed] [Google Scholar]
- 9.DiScipio RG, Daffern PJ, Schraufstatter IU, Sriramarao P. Human polymorphonuclear leukocytes adhere to complement factor H through an interaction that involves alphaMbeta2 (CD11b/CD18) J Immunol. 1998;160:4057–4066. [PubMed] [Google Scholar]
- 10.Iferroudjene D, Schouft MT, Lemercier C, Gilbert D, Fontaine M. Evidence for an active hydrophobic form of factor H that is able to induce secretion of interleukin 1-beta or by human monocytes. Eur J Immunol. 1991;21:967–972. doi: 10.1002/eji.1830210416. [DOI] [PubMed] [Google Scholar]
- 11.Yang S, Zhou M, Fowler DE, Wang P. Mechanisms of the beneficial effect of adrenomedullin and adrenomedullin-binding protein-1 in sepsis: down-regulation of proinflammatory cytokines. Crit Care Med. 2002;30:2729–2735. doi: 10.1097/00003246-200212000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Wu R, Cui X, Dong W, Zhou M, Simms HH, Wang P. Mechanisms responsible for vascular hyporesponsiveness to adrenomedullin after hemorrhage: the central role of adrenomedullin binding protein-1. Ann Surg. 2005;242:115–123. doi: 10.1097/01.sla.0000167849.10599.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang S, Zhou M, Chaudry IH, Wang P. Novel approach to prevent the transition from the hyperdynamic phase to the hypodynamic phase of sepsis: role of adrenomedullin and adrenomedullin binding protein-1. Ann Surg. 2002;236:625–633. doi: 10.1097/00000658-200211000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu R, Dong W, Zhou M, Cui X, Simms HH, Wang P. A novel approach to maintaining cardiovascular stability after hemorrhagic shock: Beneficial effects of adrenomedullin and its binding protein. Surgery. 2005;137:200–208. doi: 10.1016/j.surg.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Wattanasirichaigoon S, Menconi MJ, Delude RL, Fink MP. Effect of mesenteric ischemia and reperfusion or hemorrhagic shock on intestinal mucosal permeability and ATP content in rats. Shock. 1999;12:127–133. doi: 10.1097/00024382-199908000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Liaudet L, Szabo A, Soriano FG, Zingarelli B, Szabo C, Salzman AL. Poly (ADPribose) synthetase mediates intestinal mucosal barrier dysfunction after mesenteric ischemia. Shock. 2000;14:134–141. doi: 10.1097/00024382-200014020-00010. [DOI] [PubMed] [Google Scholar]
- 17.Saito T, Unno N, Yamamoto N, Inuzuka K, Sagara D, Suzuki M, Konno H. Intraperitoneal administration of hyperbarically oxygenated perfluorochemical enhances preservation of intestinal mucosa against ischemia/reperfusion injury. Shock. 2006;26:620–624. doi: 10.1097/01.shk.0000230297.93762.a8. [DOI] [PubMed] [Google Scholar]
- 18.Yang R, Harada T, Mollen KP, Prince JM, Levy RM, Englert JA, Gallowitsch-Puerta M, Yang L, Yang H, Tracey KJ, Harbrecht BG, Billiar TR, Fink MP. Anti-HMGB1 neutralizing antibody ameliorates gut barrier dysfunction and improves survival after hemorrhagic shock. Mol Med. 2006;12:105–114. doi: 10.2119/2006-00010.Yang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiu CJ, McArdle R, Brown HJ, Scott HJ, Gurd FN. Intestinal mucosal lesion in low flow states. Arch Surg. 1970;101:478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 20.Dwivedi AJ, Wu R, Nguyen E, Higuchi S, Wang H, Krishnasastry K, Marini CP, Ravikumar TS, Wang P. Adrenomedullin and adrenomedullin binding protein-1 prevent acute lung injury after gut ischemia-reperfusion. J Am Coll Surg. 2007;205:284–293. doi: 10.1016/j.jamcollsurg.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Sorkine P, Szold O, Halpern P, Gutman M, Greemland M, Rudick V, Goldman G. Gut decontamination reduces bowel ischemia-induced lung injury in rats. Chest. 1997;112:491–495. doi: 10.1378/chest.112.2.491. [DOI] [PubMed] [Google Scholar]
- 22.Cavriani G, Domingos HV, Soares AL, Trezena AG, Ligeiro-Oliveira AP, Oliveira-Filho RM, Sudo-Hayashi LS, Tavares de LW. Lymphatic system as a path underlying the spread of lung and gut injury after intestinal ischemia/reperfusion in rats. Shock. 2005;23:330–336. doi: 10.1097/01.shk.0000157303.76749.9b. [DOI] [PubMed] [Google Scholar]
- 23.Souba WW, Smith RJ, Wilmore DW. Glutamine metabolism by the intestinal tract. J Parenter Enteral Nutr. 1985;9:608–617. doi: 10.1177/0148607185009005608. [DOI] [PubMed] [Google Scholar]
- 24.Marin ML, Greenstein AJ, Geller SA, Gordon RE, Aufses AH., Jr A freeze fracture study of Crohn's disease of the terminal ileum: changes in epithelial tight junction organization. Am J Gastroenterol. 1983;78:537–547. [PubMed] [Google Scholar]
- 25.Swank GM, Deitch EA. Role of the gut in multiple organ failure: bacterial translocation and permeability changes. World J Surg. 1996;20:411–417. doi: 10.1007/s002689900065. [DOI] [PubMed] [Google Scholar]
- 26.Taylor DE. Revving the motor of multiple organ dysfunction syndrome. Gut dysfunction in ARDS and multiorgan failure. Respir Care Clin N Am. 1998;4:611–631. [PubMed] [Google Scholar]
- 27.Ammori BJ, Leeder PC, King RF, Barclay GR, Martin IG, Larvin M, McMahon MJ. Early increase in intestinal permeability in patients with severe acute pancreatitis: correlation with endotoxemia, organ failure, and mortality. J Gastrointest Surg. 1999;3:252–262. doi: 10.1016/s1091-255x(99)80067-5. [DOI] [PubMed] [Google Scholar]
- 28.Faries PL, Simon RJ, Martella AT, Lee MJ, Machiedo GW. Intestinal permeability correlates with severity of injury in trauma patients. J Trauma. 1998;44:1031–1035. doi: 10.1097/00005373-199806000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Doig CJ, Sutherland LR, Sandham JD, Fick GH, Verhoef M, Meddings JB. Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. Am J Respir Crit Care Med. 1998;158:444–451. doi: 10.1164/ajrccm.158.2.9710092. [DOI] [PubMed] [Google Scholar]
- 30.Cui X, Wu R, Zhou M, Dong W, Ulloa L, Yang H, Wang H, Tracey KJ, Simms HH, Wang P. Adrenomedullin and its binding protein attenuate the proinflammatory response after hemorrhage. Crit Care Med. 2005;33:391–398. doi: 10.1097/01.ccm.0000153416.41398.a9. [DOI] [PubMed] [Google Scholar]
- 31.Zhou M, Ba ZF, Chaudry IH, Wang P. Adrenomedullin binding protein-1 modulates vascular responsiveness to adrenomedullin in late sepsis. Am J Physiol Regul Integr Comp Physiol. 2002;283:R553–R560. doi: 10.1152/ajpregu.00544.2001. [DOI] [PubMed] [Google Scholar]
- 32.Ma TY, Boivin MA, Ye D, Pedram A, Said HM. Mechanism of TNF-{alpha} modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol. 2005;288:G422–G430. doi: 10.1152/ajpgi.00412.2004. [DOI] [PubMed] [Google Scholar]
- 33.Unno N, Menconi MJ, Smith M, Fink MP. Nitric oxide mediates interferon-gamma-induced hyperpermeability in cultured human intestinal epithelial monolayers. Crit Care Med. 1995;23:1170–1176. doi: 10.1097/00003246-199507000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Chavez AM, Menconi MJ, Hodin RA, Fink MP. Cytokine-induced intestinal epithelial hyperpermeability: role of nitric oxide. Crit Care Med. 1999;27:2246–2251. doi: 10.1097/00003246-199910000-00030. [DOI] [PubMed] [Google Scholar]
- 35.Adams RB, Planchon SM, Roche JK. IFN-gamma modulation of epithelial barrier function. Time course, reversibility, and site of cytokine binding. J Immunol. 1993;150:2356–2363. [PubMed] [Google Scholar]
- 36.Diebel LN, Liberati DM, Baylor AE, III, Brown WJ, Diglio CA. The pivotal role of tumor necrosis factor-alpha in signaling apoptosis in intestinal epithelial cells under shock conditions. J Trauma. 2005;58:995–1001. doi: 10.1097/01.ta.0000162727.30897.c8. [DOI] [PubMed] [Google Scholar]
- 37.Ye D, Ma I, Ma TY. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2006;290:G496–G504. doi: 10.1152/ajpgi.00318.2005. [DOI] [PubMed] [Google Scholar]
- 38.Luyer MD, Buurman WA, Hadfoune M, Jacobs JA, Konstantinov SR, Dejong CH, Greve JW. Pretreatment with high-fat enteral nutrition reduces endotoxin and tumor necrosis factor-alpha and preserves gut barrier function early after hemorrhagic shock. Shock. 2004;21:65–71. doi: 10.1097/01.shk.0000101671.49265.cf. [DOI] [PubMed] [Google Scholar]
- 39.Wu R, Zhou M, Wang P. Adrenomedullin and adrenomedullin binding protein-1 downregulate TNF-alpha in macrophage cell line and rat Kupffer cells. Regul Pept. 2003;112:19–26. doi: 10.1016/s0167-0115(03)00018-1. [DOI] [PubMed] [Google Scholar]
- 40.Goldman G, Soffer D, Heller L, Aderka D, Lahat A, Klausner JM. Tumour necrosis factor mediates bacterial translocation after haemorrhagic shock and endotoxaemia. Eur J Surg. 2001;167:299–304. doi: 10.1080/110241501300091543. [DOI] [PubMed] [Google Scholar]