Abstract
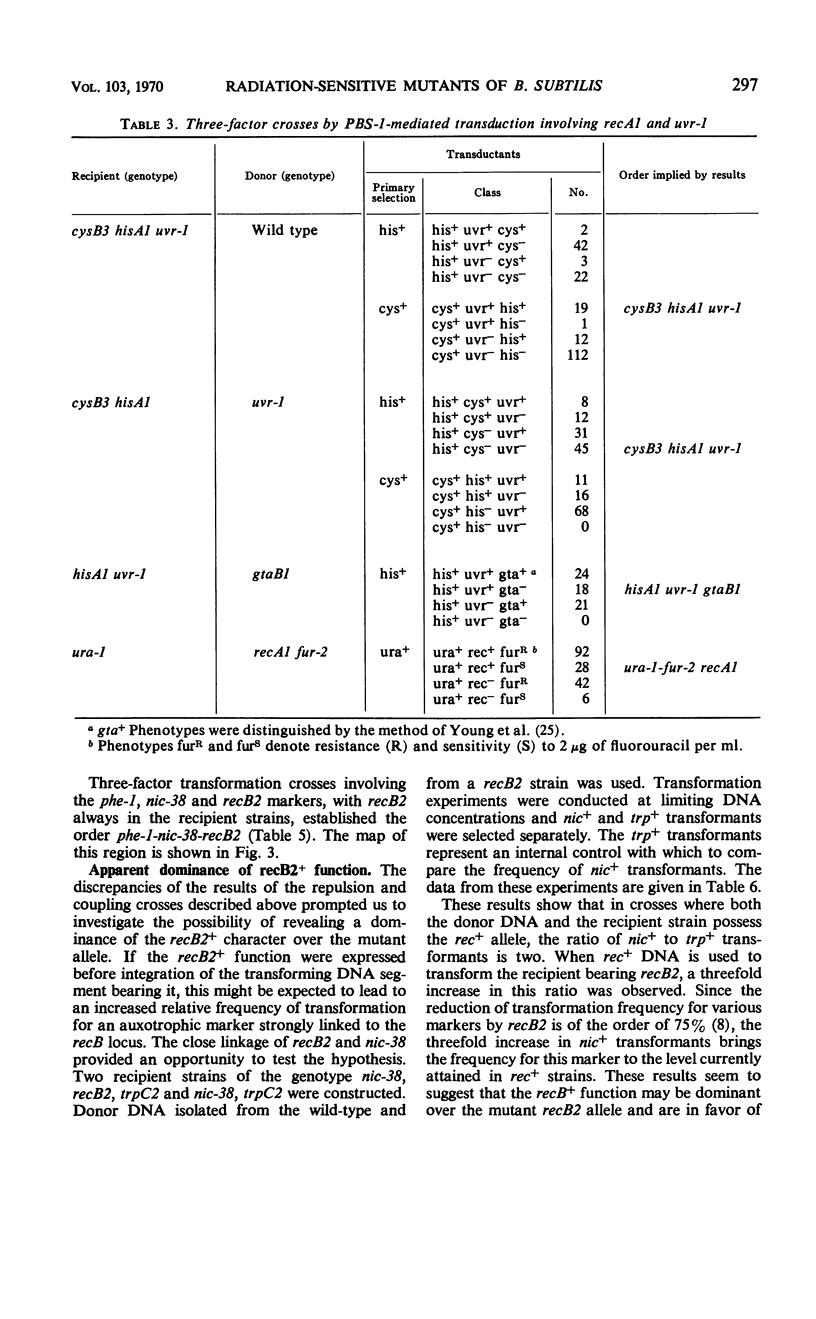
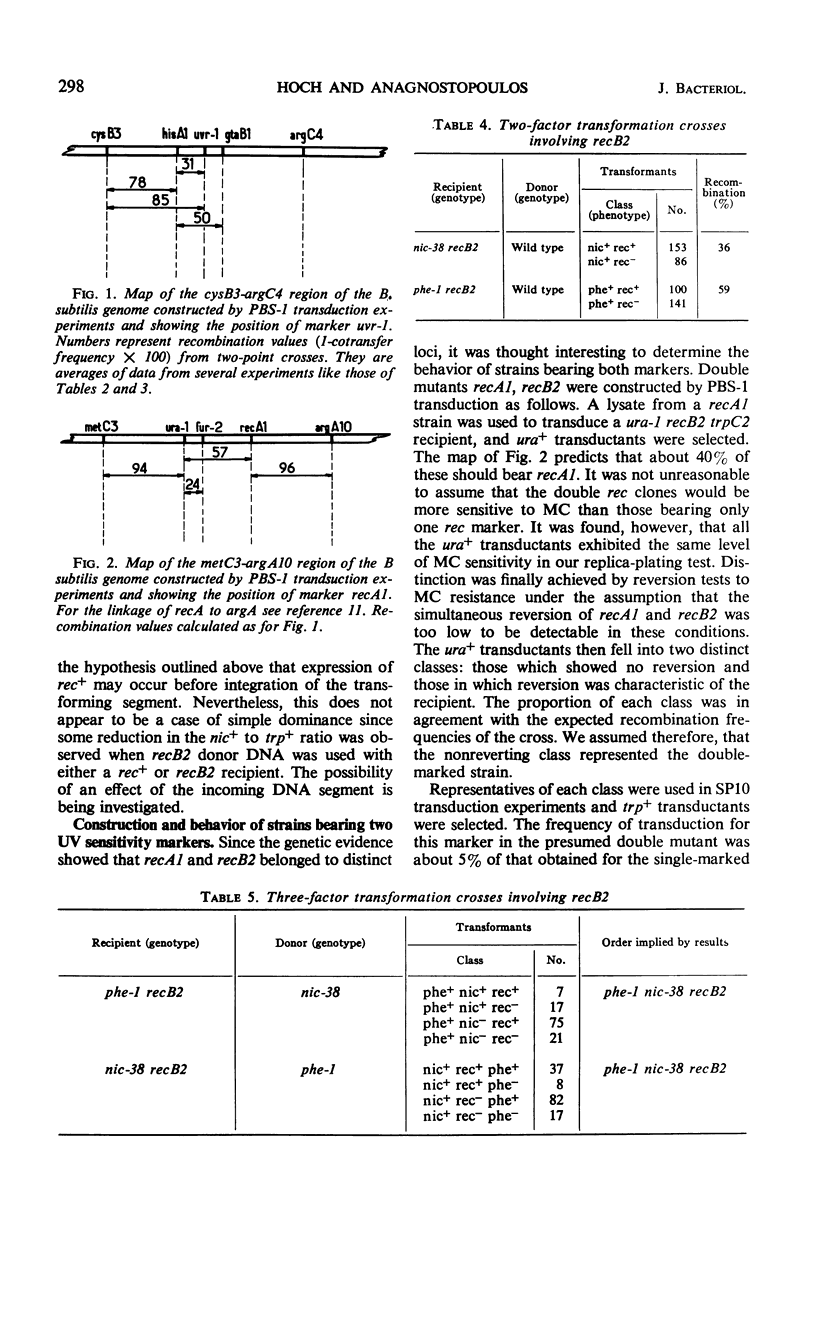
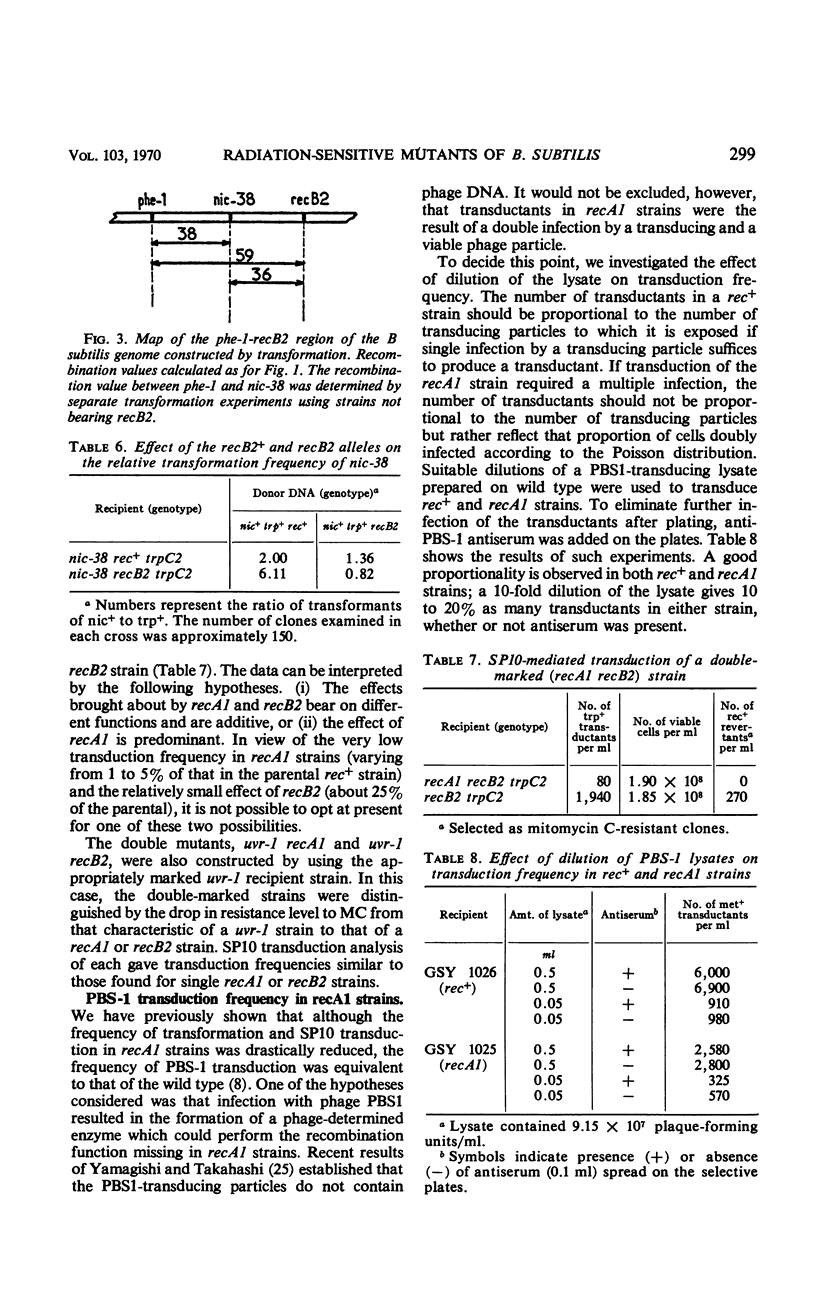
Transformation and transduction crosses involving recA1, recB2, and urv-1 mutations have shown that these mutations belong to three distinct unlinked genetic loci. The precise position of these loci on the Bacillus subtilis chromosome map has been determined. The behavior of recB2 strains in transformation studies suggested a dominance of recB2+ function over recB2 and an early expression of this phenotype during transformation. Strains bearing two ultraviolet sensitivity markers possess a phenotype characteristic of the marker with the most adverse effect on recombination. The possibility that the effects of the two mutations are additive was also considered. Results are also presented which show that a phage-induced enzyme is not responsible for the high transducibility of recA1 strains.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARAT M., ANAGNOSTOPOULOS C., SCHNEIDER A. M. LINKAGE RELATIONSHIPS OF GENES CONTROLLING ISOLEUCINE, VALINE, AND LEUCINE BIOSYNTHESIS IN BACILLUS SUBTILIS. J Bacteriol. 1965 Aug;90:357–369. doi: 10.1128/jb.90.2.357-369.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J. The beginning of a genetic analysis of recombination proficiency. J Cell Physiol. 1967 Oct;70(2 Suppl):165–180. doi: 10.1002/jcp.1040700412. [DOI] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donch J., Greenberg J. Loci of radiation sensitivity in Bs strains of Escherichia coli. Genet Res. 1968 Apr;11(2):183–191. doi: 10.1017/s0016672300011356. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R., Smith I. Transformation and transduction in Bacillus subtilis: evidence for separate modes of recombinant formation. J Mol Biol. 1969 Oct 28;45(2):155–179. doi: 10.1016/0022-2836(69)90097-7. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Goldthwaite C., Smith I., Marmur J. Genetic mapping in Bacillus subtilis. J Mol Biol. 1967 Jul 14;27(1):163–185. doi: 10.1016/0022-2836(67)90358-0. [DOI] [PubMed] [Google Scholar]
- Emmerson P. T. Recombination deficient mutants of Escherichia coli K12 that map between thy A and argA. Genetics. 1968 Sep;60(1):19–30. doi: 10.1093/genetics/60.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A., Barat M., Anagnostopoulos C. Transformation and transduction in recombination-defective mutants of Bacillus subtilis. J Bacteriol. 1967 Jun;93(6):1925–1937. doi: 10.1128/jb.93.6.1925-1937.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P. Genes that control DNA repair and genetic recombination in Escherichia coli. Adv Biol Med Phys. 1968;12:299–317. doi: 10.1016/b978-1-4831-9928-3.50016-3. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L., Stedeford J. B. Some properties of excision-defective recombination-deficient mutants of Escherichia coli K-12. J Bacteriol. 1969 Mar;97(3):1134–1141. doi: 10.1128/jb.97.3.1134-1141.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Hegarat J. C., Anagnostopoulos C. Localisation chromosomique d'un gène gouvernant la synthèse d'une phosphatase alcaline chez Bacillus subtilis. C R Acad Sci Hebd Seances Acad Sci D. 1969 Nov 17;269(20):2048–2050. [PubMed] [Google Scholar]
- Mahler I., Grossman L. Transformation of radiation sensitive strains of Micrococcus lysodeikticus. Biochem Biophys Res Commun. 1968 Sep 6;32(5):776–781. doi: 10.1016/0006-291x(68)90307-0. [DOI] [PubMed] [Google Scholar]
- Moseley B. E. Repair of ultraviolet radiation damage in sensitive mutants of Micrococcus radiodurans. J Bacteriol. 1969 Feb;97(2):647–652. doi: 10.1128/jb.97.2.647-652.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata N., Ikeda Y. Inactivation of transforming DNA by ultraviolet irradiation: a study with ultraviot-sensitive mutants of Bacillus subtilis. Mutat Res. 1969 Mar-Apr;7(2):133–139. doi: 10.1016/0027-5107(69)90025-6. [DOI] [PubMed] [Google Scholar]
- Okubo S., Nakayama H. DNA synthesis after ultraviolet light irradiation in uv-sensitive mutants of Bacillus subtilis. Mutat Res. 1967 Sep-Oct;4(5):533–541. doi: 10.1016/0027-5107(67)90039-5. [DOI] [PubMed] [Google Scholar]
- Okubo S., Romig W. R. Impaired transformability of Bacillus subtilis mutant sensitive to mitomycin C and ultraviolet radiation. J Mol Biol. 1966 Feb;15(2):440–454. doi: 10.1016/s0022-2836(66)80120-1. [DOI] [PubMed] [Google Scholar]
- Pauling C., Hamm L. Properties of a temperature-sensitive radiation-sensitive mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1495–1502. doi: 10.1073/pnas.60.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter H., Strauss B. Repair of damage induced by a monofunctional alkylating agent in a transformable, ultraviolet-sensitive strain of Bacillus subtilis. J Mol Biol. 1965 Nov;14(1):179–194. doi: 10.1016/s0022-2836(65)80239-x. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Brown D. C., Boling M. E., Mattingly A., Gordon M. P. Repair of deoxyribonucleic acid in Haemophilus influenzae. I. X-ray sensitivity of ultraviolet-sensitive mutants and their behavior as hosts to ultraviolet-irradiated bacteriophage and transforming deoxyribonucleic acid. J Bacteriol. 1968 Feb;95(2):546–558. doi: 10.1128/jb.95.2.546-558.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skavronskaya A. G., Pokrovsky V. N., Zlatev V. I., Andreeva I. V. Isolation and some properties of the radiation-sensitive mutant of S. typhimurium LT 2. Mutat Res. 1969 Mar-Apr;7(2):248–251. doi: 10.1016/0027-5107(69)90038-4. [DOI] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J., Low B. Genetic location of certain mutations conferring recombination deficiency in Escherichia coli. J Bacteriol. 1969 Jan;97(1):244–249. doi: 10.1128/jb.97.1.244-249.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing J. P., Levine M., Smith H. O. Recombination-deficient mutant of Salmonella typhimurium. J Bacteriol. 1968 May;95(5):1828–1834. doi: 10.1128/jb.95.5.1828-1834.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi H., Takahashi I. Transducing particles of PBS 1. Virology. 1968 Dec;36(4):639–645. doi: 10.1016/0042-6822(68)90194-3. [DOI] [PubMed] [Google Scholar]
- Young F. E., Smith C., Reilly B. E. Chromosomal location of genes regulating resistance to bacteriophage in Bacillus subtilis. J Bacteriol. 1969 Jun;98(3):1087–1097. doi: 10.1128/jb.98.3.1087-1097.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Putte P., Holloway B. W. A thermosensitive recombination deficient mutant of Pseudomonas aeruginosa. Mutat Res. 1968 Sep-Oct;6(2):195–203. doi: 10.1016/0027-5107(68)90034-1. [DOI] [PubMed] [Google Scholar]


