Abstract
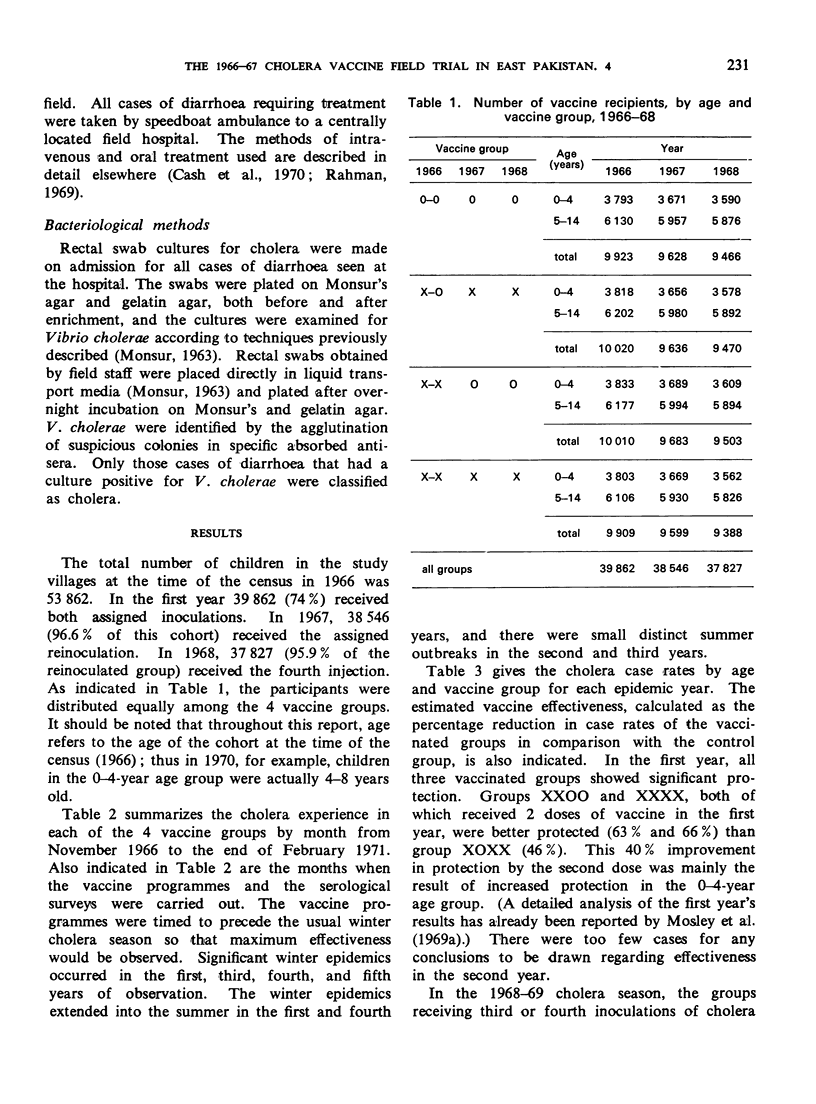
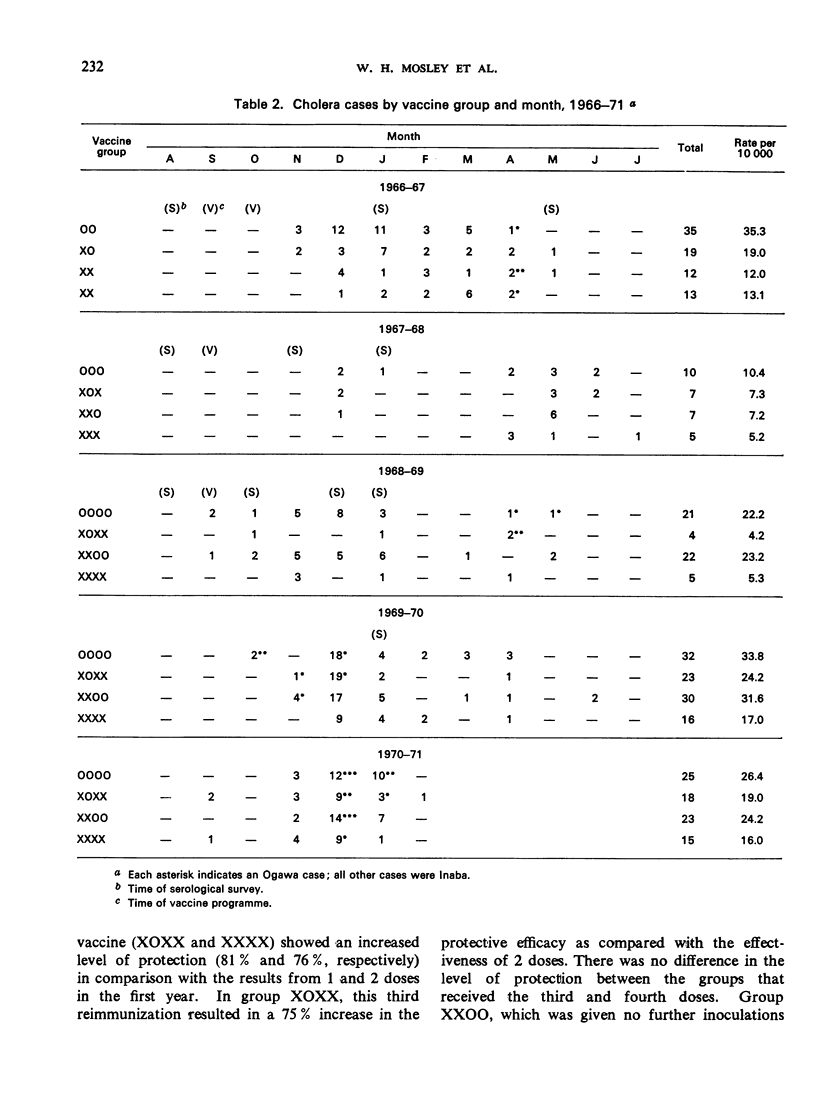
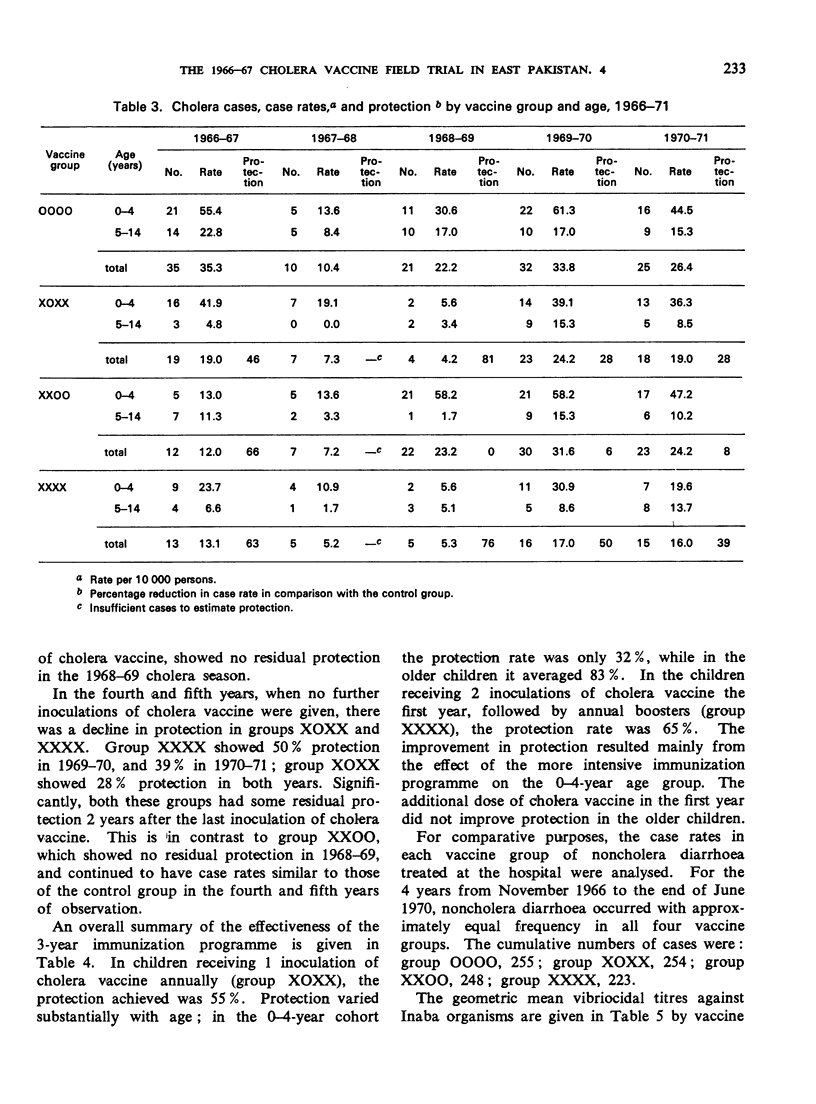
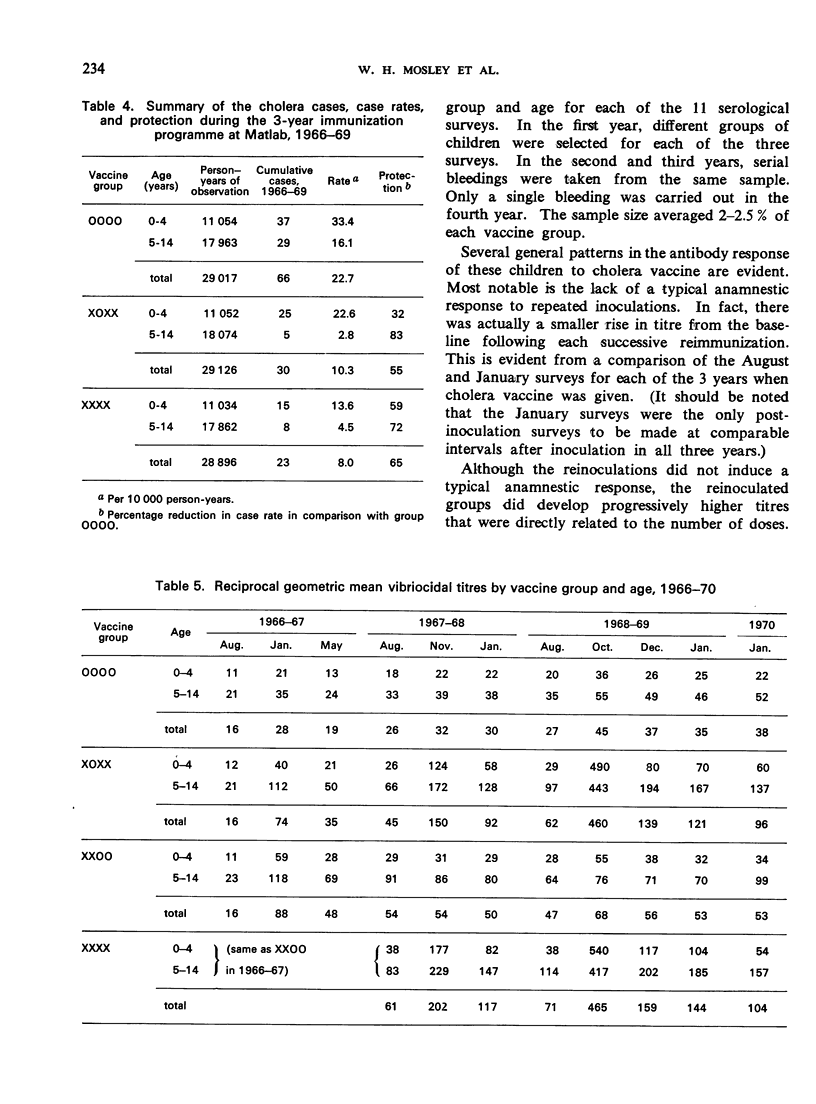
A controlled cholera vaccine field trial was carried out in rural East Pakistan to determine the efficacy of a cholera vaccine of average antigenic potency when used in a continuing programme with annual reimmunizations. A cohort of 40 000 children aged 0-14 years was equally divided into a control group and 3 vaccine groups. Inoculations of vaccine were given annually for 3 years just before the start of the cholera season, and follow-up continued for 2 additional years. The results indicate that there was increasing protection with reimmunization, reaching a maximum with 3 doses. One dose produced 43% protection, 2 doses 64%, 3 doses 81%, and 4 doses 76%. Protection was more sustained after reimmunization; being 50% and 39%, 1 and 2 years after the fourth injection, respectively. Serological surveys suggested a general parallel in the antibody response to vaccine and the level of protection achieved; however, the levels of vibriocidal antibody titres could not be related directly to levels of protection. The overall protection achieved with the 3-year programme of annual reimmunizations was 55% for the group receiving one inoculation annually, and 65% for the group receiving 2 inoculations in the first year followed by annual reimmunizations. When the costs and effectiveness of annual vaccine programmes are compared with those for cholera treatment centres, it becomes clear that the cholera vaccines now available are not appropriate alternatives to treatment in routine cholera control programmes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benenson A. S., Mosley W. H., Fahimuddin M., Oseasohn R. O. Cholera vaccine field trials in east Pakistan. 2. Effectiveness in the field. Bull World Health Organ. 1968;38(3):359–372. [PMC free article] [PubMed] [Google Scholar]
- Benenson A. S., Saad A., Mosley W. H. Serological studies in cholera. 2. The vibriocidal antibody response of cholera patients determined by a microtechnique. Bull World Health Organ. 1968;38(2):277–285. [PMC free article] [PubMed] [Google Scholar]
- Cash R. A., Nalin D. R., Rochat R., Reller L. B., Haque Z. A., Rahman A. S. A clinical trial of oral therapy in a rural cholera-treatment center. Am J Trop Med Hyg. 1970 Jul;19(4):653–656. doi: 10.4269/ajtmh.1970.19.653. [DOI] [PubMed] [Google Scholar]
- Gangarosa E. J., DeWitt W. E., Feeley J. C., Adams M. R. Significance of vibriocidal antibodies with regard to immunity to cholera. J Infect Dis. 1970 May;121(Suppl):36+–36+. doi: 10.1093/infdis/121.supplement.s36. [DOI] [PubMed] [Google Scholar]
- Mosley W. H., Benenson A. S., Barui R. A serological survey for cholear antibodies in rural east Pakistan. 1. The distribution of antibody in the control population of a cholera-vaccine field-trial area and the relation of antibody titre to the pattern of endemic cholera. Bull World Health Organ. 1968;38(3):327–334. [PMC free article] [PubMed] [Google Scholar]
- OSEASOHN R. O., BENENSON A. S., FAHIMUDDIN M. FIELD TRIAL OF CHOLERA VACCINE IN RURAL EAST PAKISTAN. FIRST YEAR OF OBSERVATION. Lancet. 1965 Feb 27;1(7383):450–452. doi: 10.1016/s0140-6736(65)91586-2. [DOI] [PubMed] [Google Scholar]
- PITTMAN M., FEELEY J. C. Protective activity of cholera vaccines against E1 Tor cholera vibrios. Bull World Health Organ. 1963;28(3):379–383. [PMC free article] [PubMed] [Google Scholar]


