Abstract
Insecticide resistance is an inherent characteristic dependent on relatively simple genetic mechanisms. This seems to be especially true of dieldrin resistance in anopheline mosquitos, though less obvious in DDT resistance among these species; little is known as yet about the inheritance of organophosphate and carbamate resistance as it occurs in Anopheles albimanus. The speed of selection of resistance depends on the original frequency of the gene or genes involved, the nature of the resistance imparted, and the selection pressure of the insecticide. This in turn depends on the inherent toxicity of the chemical, the efficiency with which it is applied, the proportion of the mosquito population coming under its influence, and the behaviour of the mosquito. In the past, too much reliance has been placed on the determination of the LD50 in assessing the presence or absence of insecticide resistance. Quite high incidences of resistant individuals can result in such small changes in the LD50 that resistance may be overlooked. The use of single discriminating dosages is advocated, based on concentrations of insecticides that normally kill all susceptible individuals. The authors discuss such dosages in respect of dieldrin and DDT, and put forward newly-established tentative discriminating dosages for organophosphorus and carbamate insecticides, which await field confirmation.
From a practical standpoint, an insecticide should not be abandoned or replaced by another as soon as resistance is confirmed. This may not be necessary where the degree of resistance is not high and the vector is not highly efficient. Certain procedures are proposed in order to assess the epidemiological and entomological implications of resistance before the insecticide concerned is abandoned.
Full text
PDF

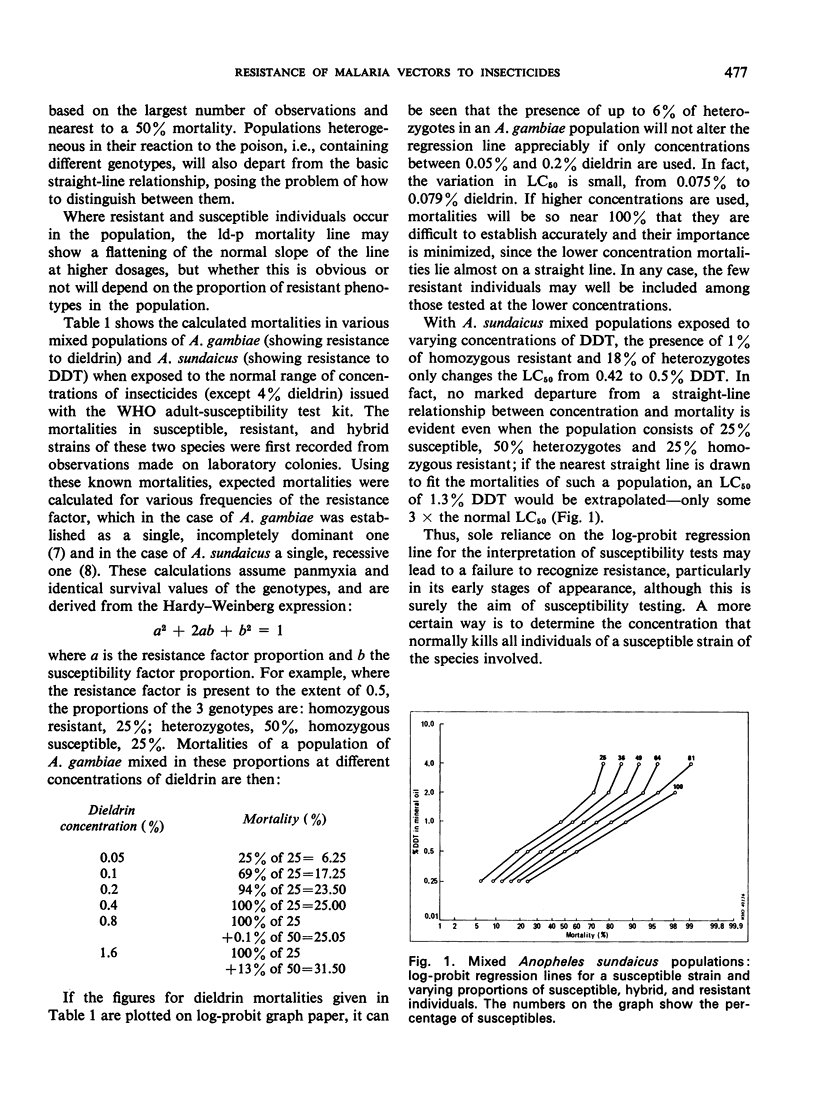
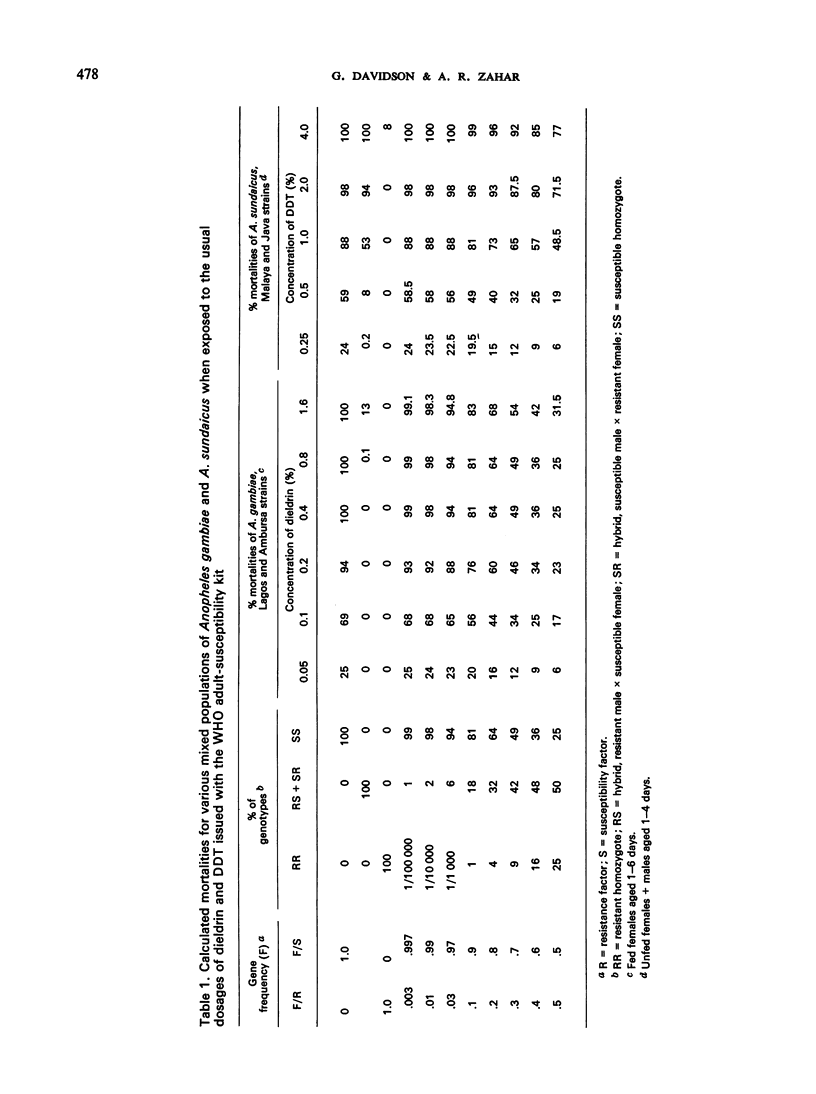





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariaratnam V., Georghiou G. P. Selection for resistance to carbamate and organophoshorus insecticides in Anopheles albimanus. Nature. 1971 Aug 27;232(5313):642–644. doi: 10.1038/232642a0. [DOI] [PubMed] [Google Scholar]
- Breeland S. G., Kliewer J. W., Austin J. R., Miller C. W. Observations on malathion-resistant adults of Anopheles albimanus Wiedemann in coastal El Salvador. Bull World Health Organ. 1970;43(4):627–631. [PMC free article] [PubMed] [Google Scholar]
- DAVIDSON G. Insecticide resistance in Anopheles gambiae Giles: a case of simple mendelian inheritance. Nature. 1956 Oct 20;178(4538):863–864. doi: 10.1038/178863a0. [DOI] [PubMed] [Google Scholar]
- DAVIDSON G. Insecticide resistance in Anopheles sundaicus. Nature. 1957 Dec 14;180(4598):1333–1335. doi: 10.1038/1801333a0. [DOI] [PubMed] [Google Scholar]
- Georghiou G. P., Ariaratnam V., Breeland S. G. Development of resistance to carbamates and organophosphorus compounds in Anopheles albimanus in nature. Bull World Health Organ. 1972;46(4):551–554. [PMC free article] [PubMed] [Google Scholar]
- LUEN S. C., SHALABY A. M. Preliminary note on the development of DDT-resistance in Anopheles culicifacies Giles in Panchmahals District, Gujerat State, India. Bull World Health Organ. 1962;26:128–134. [PMC free article] [PubMed] [Google Scholar]
- PEFFLY R. L. Insecticide resistance in anophelines in eastern Saudi Arabia. Bull World Health Organ. 1959;20:757–776. [PMC free article] [PubMed] [Google Scholar]


