Abstract
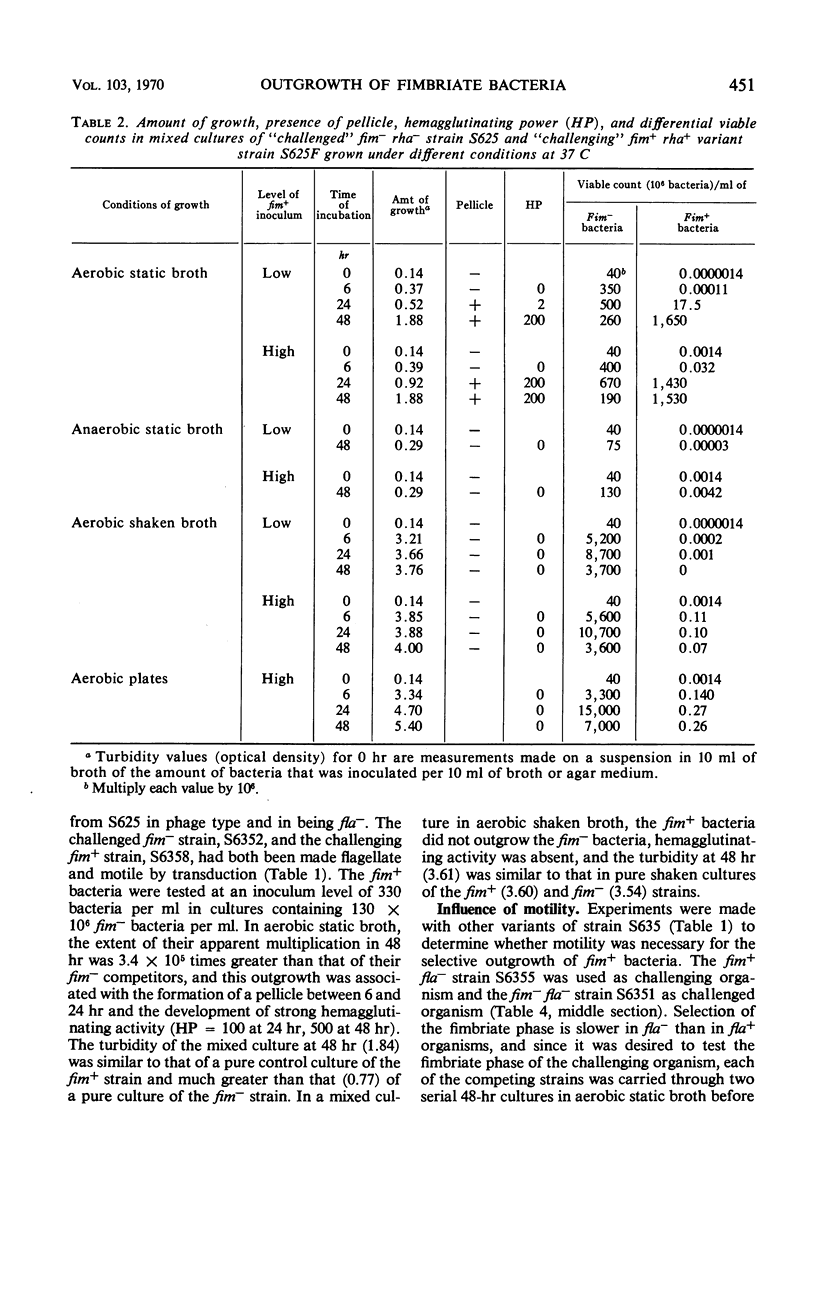
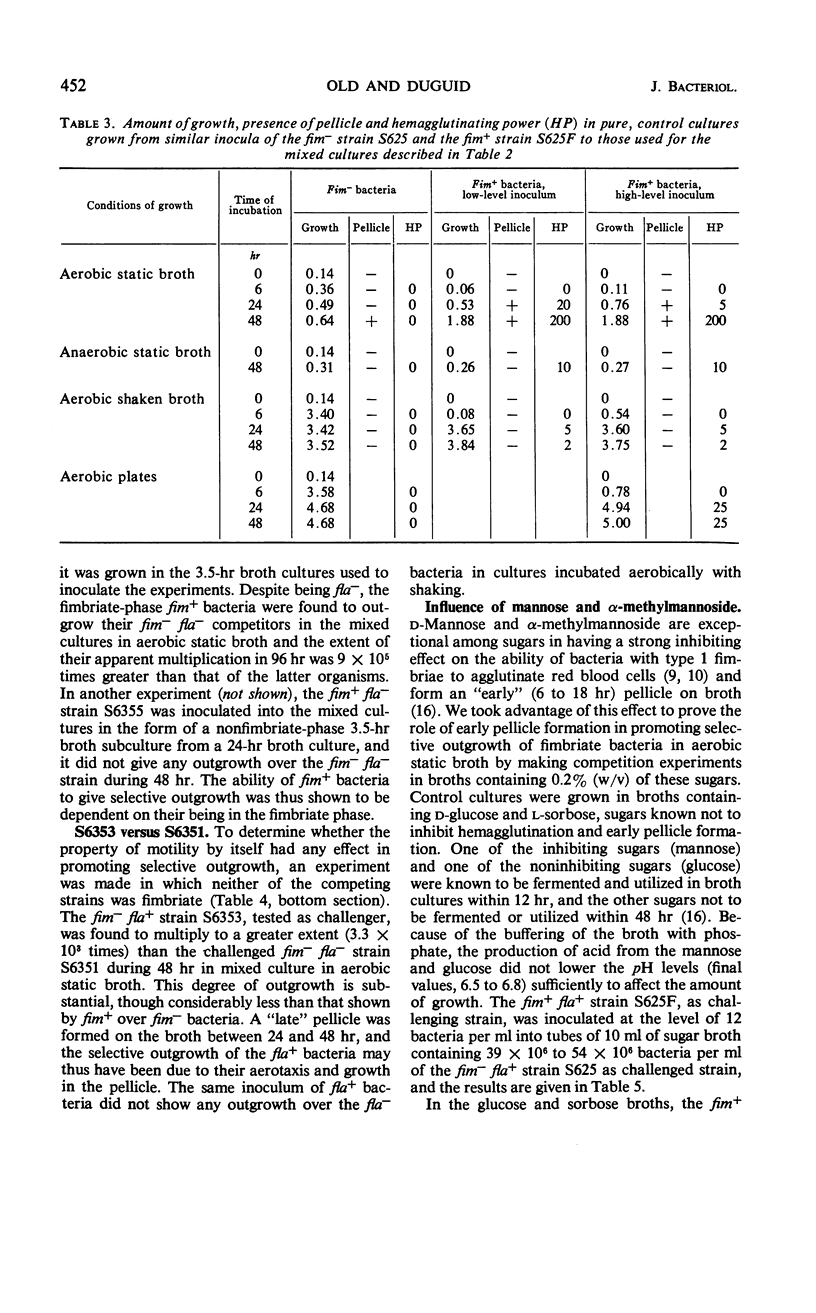
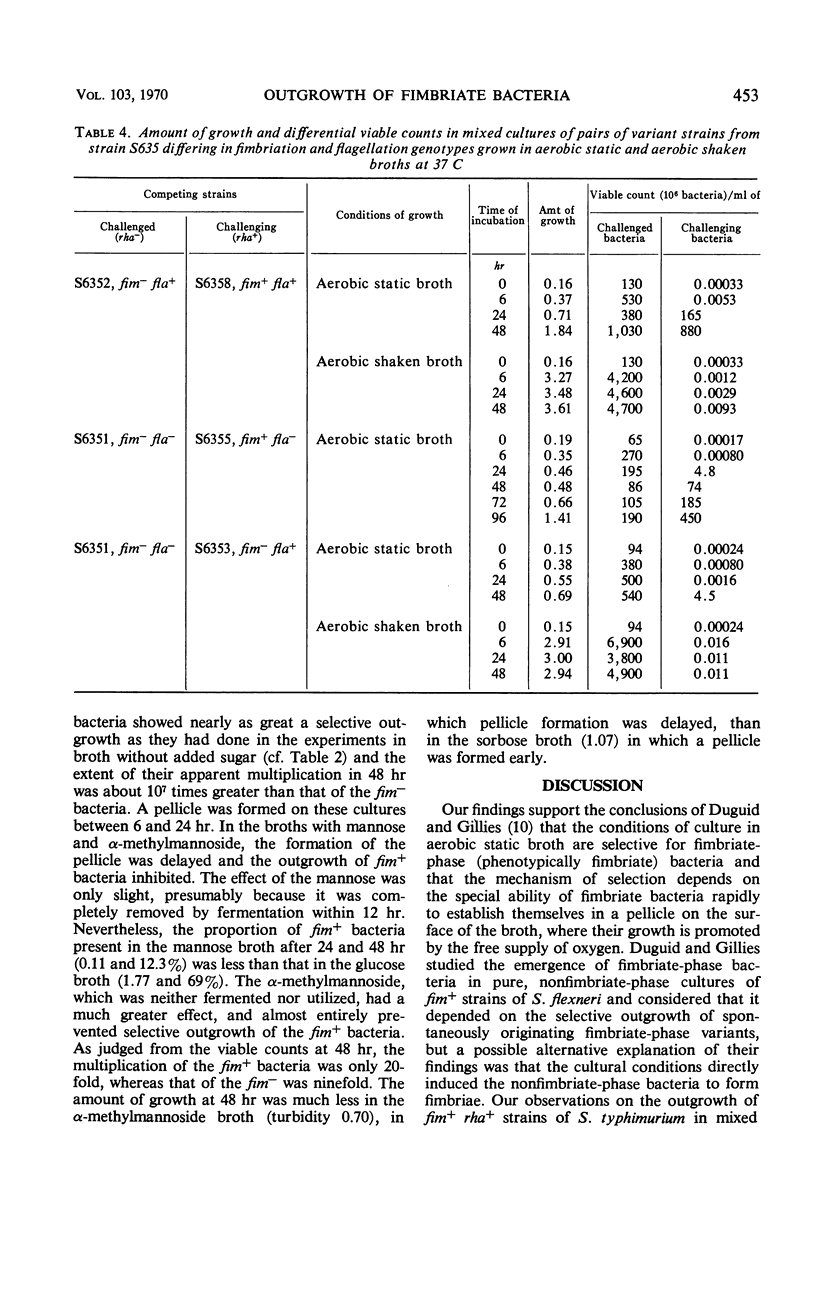
Competitive mixed cultures were grown from inocula of a large number of bacteria of a genotypically nonfimbriate (fim−) strain of Salmonella typhimurium and a small number of a genotypically fimbriate (fim+) variant strain that formed type 1 fimbriae and had been derived from the fim− strain by phage transduction. The fim+ strain differed from the fim− strain in fermenting l-rhamnose (rha+), and the viable fim+ and fim− bacteria present in the cultures after different periods at 37 C were counted differentially in platings on rhamnose media. When the cultures were grown under aerobic static conditions in tubes of nutrient broth, the fim+ bacteria rapidly outgrew the fim− bacteria, so that, although starting as a small minority (e.g., 1 in 107), they approached or surpassed the number of the fim− in 48 hr. A pellicle consisting of fimbriate bacteria was formed on the surface of the broth between 6 and 24 hr, and it is thought that the advantage of access to atmospheric oxygen enjoyed by these bacteria in the pellicle enabled them to outgrow the fim− bacteria confined in the oxygen-depleted broth. The fim+ bacteria did not show selective outgrowth in mixed cultures grown in broth aerated by continuous shaking, in static broth incubated anaerobically in hydrogen, and on aerobic agar plates, i.e., under conditions not allowing an advantage from pellicle formation. The outgrowth of fim+ bacteria in aerobic static broth was prevented by the addition of α-methylmannoside, a substance that inhibits the adhesive and early pellicle-forming properties of bacteria with type 1 fimbriae. A motile flagellate (fla+) variant of a fim−fla− strain of S. typhimurium outgrew its parent strain in mixed cultures in aerobic static broth, but the selective advantage conferred by motility was weaker than that conferred by fimbriation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARACCHINI O., SHERRIS J. C. The chemotactic effect of oxygen on bacteria. J Pathol Bacteriol. 1959 Apr;77(2):565–574. doi: 10.1002/path.1700770228. [DOI] [PubMed] [Google Scholar]
- BOYD J. S. Immunity of lysogenic bacteria. Nature. 1956 Jul 21;178(4525):141–141. doi: 10.1038/178141a0. [DOI] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- CALLOW B. R. A new phage-typing scheme for Salmonella typhi-murium. J Hyg (Lond) 1959 Sep;57:346–359. doi: 10.1017/s0022172400020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUGUID J. P., SMITH I. W., DEMPSTER G., EDMUNDS P. N. Non-flagellar filamentous appendages (fimbriae) and haemagglutinating activity in Bacterium coli. J Pathol Bacteriol. 1955 Oct;70(2):335–348. doi: 10.1002/path.1700700210. [DOI] [PubMed] [Google Scholar]
- Duguid J. P., Anderson E. S., Campbell I. Fimbriae and adhesive properties in Salmonellae. J Pathol Bacteriol. 1966 Jul;92(1):107–138. doi: 10.1002/path.1700920113. [DOI] [PubMed] [Google Scholar]
- Duguid J. P., Anderson E. S. Terminology of bacterial fimbriae, or pili, and their types. Nature. 1967 Jul 1;215(5096):89–90. doi: 10.1038/215089a0. [DOI] [PubMed] [Google Scholar]
- Duguid J. P. The function of bacterial fimbriae. Arch Immunol Ther Exp (Warsz) 1968;16(2):173–188. [PubMed] [Google Scholar]
- GILLIES R. R. COLICINE PRODUCTION AS AN EPIDEMIOLOGICAL MARKER OF SHIGELLA SONNEI. J Hyg (Lond) 1964 Mar;62:1–9. doi: 10.1017/s0022172400039711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenroth A., Duguid J. P. Demonstration of different mutational sites controlling rhamnose fermentation in FIRN and non-FIRN rha-strains of Salmonella typhimurium: an essay in bacterial archaeology. Genet Res. 1968 Apr;11(2):151–169. doi: 10.1017/s0016672300011320. [DOI] [PubMed] [Google Scholar]
- Old D. C., Corneil I., Gibson L. F., Thomson A. D., Duguid J. P. Fimbriation, pellicle formation and the amount of growth of salmonellas in broth. J Gen Microbiol. 1968 Apr;51(1):1–16. doi: 10.1099/00221287-51-1-1. [DOI] [PubMed] [Google Scholar]
- STOCKER B. A. Transduction of flagellar characters in Salmonella. J Gen Microbiol. 1953 Dec;9(3):410–433. doi: 10.1099/00221287-9-3-410. [DOI] [PubMed] [Google Scholar]
- Smith J. L., Doetsch R. N. Studies on negative chemotaxis and the survival value of motility in Pseudomonas fluorescens. J Gen Microbiol. 1969 Mar;55(3):379–391. doi: 10.1099/00221287-55-3-379. [DOI] [PubMed] [Google Scholar]
- ZINDER N. D., LEDERBERG J. Genetic exchange in Salmonella. J Bacteriol. 1952 Nov;64(5):679–699. doi: 10.1128/jb.64.5.679-699.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]


