Abstract
Cytochrome c oxidase (CcO) is a multimetallic enzyme that carries out the reduction of O2 to H2O and is essential to respiration, providing the energy that powers all aerobic organisms by generating heat and forming ATP. The oxygen-binding heme a3 should be subject to fatal inhibition by chemicals that could compete with O2 binding. Near the CcO active site is another enzyme, NO synthase, which produces the gaseous hormone NO. NO can strongly bind to heme a3, thus inhibiting respiration. However, this disaster does not occur. Using functional models for the CcO active site, we show how NO inhibition is avoided; in fact, it is found that NO can protect the respiratory enzyme from other inhibitors such as cyanide, a classic poison.
Keywords: amyl nitrite, cyanide poisoning, synthetic functional model, EPR
In this article we answer an important question: why is the active site of the respiratory enzyme cytochrome c oxidase (CcO) not severely inhibited by the diffusing hormone NO, produced by a neighboring NO synthase (NOS)? Moreover, we demonstrate that NO should protect CcO from other inhibitors.
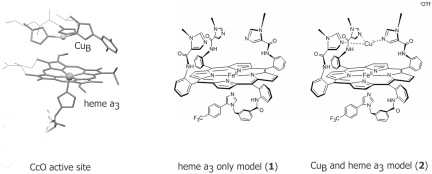
CcO is a multicomponent membrane protein that catalyzes the 4e− reduction of O2 to H2O in all eukaryotes (1). The energy released in this process is used to produce a proton gradient across the membrane, which provides the driving force for ATP synthesis. The active site of CcO contains a bimetallic center comprising an iron porphyrin heme a3 and a tris-histidine-coordinated Cu (CuB) in the distal pocket (Fig. 1) (2, 3). During catalytic turnover, the FeII/CuI center reduces O2, and the resulting oxidized site is reduced via electron transfer from two electron donors, heme a and CuA, regenerating the FeII/CuI site. CcO is strongly inhibited by high levels of nitric oxide (NO) (KI = 0.27 μM), carbon monoxide (CO) (KI = 0.32 μM), and cyanide (CN−) (KI = 0.2 μM) (4). NO is produced continuously in the mitochondrial membrane adjacent to CcO by mitochondrial NOS (5, 6). Apart from car exhaust and cigarette smoke, CN− is produced naturally in the human body as a metabolic by-product of cyanogenic glycosides that are present in bitter almonds, apple seeds, raw yucca, and cassava roots (7). CO is produced in cells as a by-product of heme degradation (8). Any sudden increase in the flux of these inhibitors leads to a surge of partially reduced oxygen species in the cell, which eventually leads to cell death (9). NO has a high equilibrium binding constant (Kd < 10−9 M) and a fast second-order kinetic rate constant (kon = ≈108 M−1·s−1) for reduced ferrous porphyrin active sites, including the reduced heme a3 site of CcO (10, 11). Because of a constant flux of NO at the CcO active site ([O2]/[NO] = 1,000 in mitochondria), there must be a pathway to avoid inhibition. Inhibition of CcO by NO has been reported to be reversible in the presence of O2; that is, after the initial inhibition by NO, the O2-reducing activity of CcO is slowly regained in the presence of O2 (11–13). From optical studies of the interaction of oxymyoglobin (Mb-O2) with NO-bound CcO, Sarti et al. (14, 15) proposed a mechanism that entails dissociation of bound NO from the heme a3 site. However, this proposal presents a dilemma, because dissociation of NO from heme a3 has a first-order rate of koff = 0.004 s−1, which is orders of magnitude slower than the rate necessary for normal CcO turnover. Pearce et al. (16) have investigated the same reaction by using more quantitative probes (e.g., EPR spectroscopy) and proposed that in the presence of O2, the NO is catabolized to nitrite, analytically detected, in the active site of CcO. This mechanism invoked generation of superoxide (O2−) by oxidation of CuB by O2 near an NO-bound heme a3 site, which then decays via a peroxynitrite intermediate. Although this seems reasonable, it is hard to investigate this mechanism in a protein active site because of the presence of several other chromophores. CO also has an affinity for the catalytically active reduced FeII/CuI form of CcO (Kd = 10−6 M) and competes with O2 binding to this active site (17). A classic inhibitor of CcO, CN− binds tightly to the fully oxidized site (Kd = 10−6 M), resulting in the formation of a low-spin heme a3 center (18). This binding shifts the reduction potential (E°) of heme a3 to +150 mV (E° = +350 mV resting), which should jeopardize the reduction of the fully oxidized FeIII/CuII form to the catalytically active FeII/CuI form by the adjacent heme a center (E° = 250–300 mV), which could also inhibit the enzyme turnover (19). The common antidote for CN− poisoning is to administer organic nitrites [e.g., amyl nitrite (AmN)], thiosulfate, and plentiful oxygen. In this article we use a functional synthetic model complex of CcO in its monometallic Fe-only form (1) or in its functional bimetallic Fe/Cu form (2) to study the interaction of NO with these inhibitors and suggest a mechanism of recovery from each inhibitor (20–22).
Fig. 1.
The CcO active site along with the electron-transfer heme a site and model complexes 1 and 2.
Results and Discussion
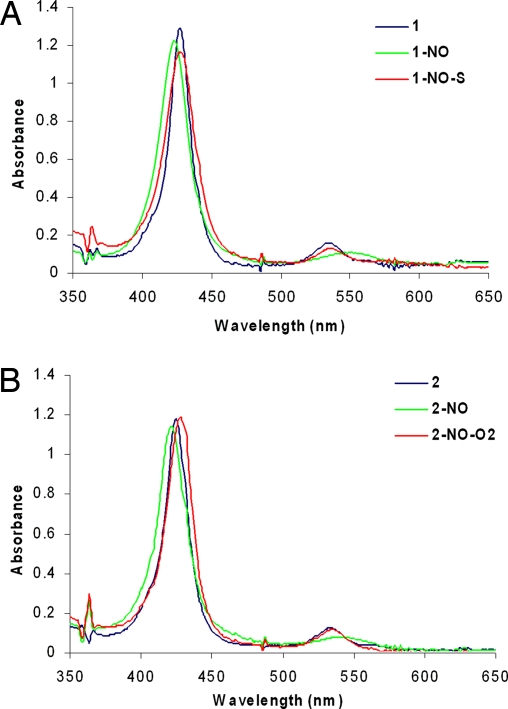
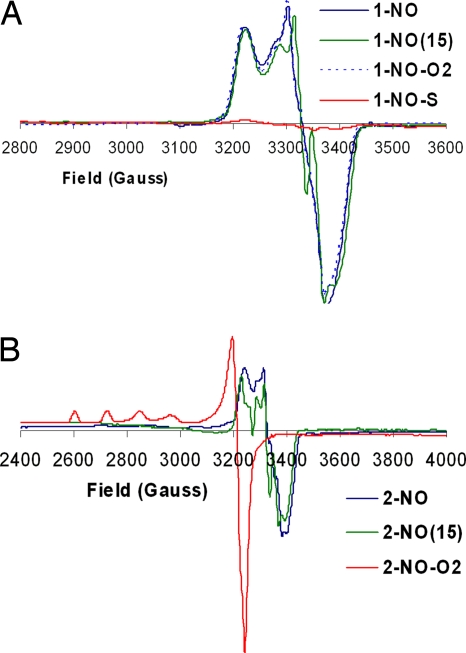
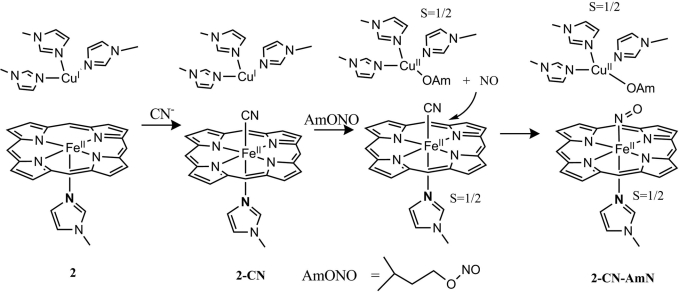
The mononitrosyl complexes 1-NO and 2-NO were prepared before studying reactivity with O2 and/or O2−. These NO complexes were generated by addition of 1 eq of NO (Scheme 1) to their respective precursors. Their absorption spectra show significant shifts in the absorption bands: 428 nm (Soret) and 536 nm to 425 nm (Soret) and 548 nm, respectively (Fig. 2). The EPR of both 1-NO and 2-NO (Fig. 3) is characteristic of low-spin six-coordinate S = 1/2 iron-nitrosyl species with gz = 2.078, gy = 2.015, and gx = 1.97. The multiple 14N superhyperfine in the Ay (NImz = 6 cm−1, NNO = 22 cm−1) and its perturbation by isotopically enriched 15NO (NImz = 6 cm−1, NNO = 31 cm−1) are similar to data previously reported for CcO's active site and six-coordinate iron nitrosyl with imidazole axial ligands [supporting information (SI) Fig. S1] (23, 24). Addition of excess O2 to 1-NO, even after prolonged exposure, does not result in any change, neither in the UV-visible (UV-Vis) nor in the EPR spectra (1-NO-O2; Figs. 2A and 3A). However, with 2-NO, addition of O2 shifts of the absorption maxima to 429 and 539 nm (Fig. 2B), which is similar to that of the ferrous form. Simultaneous EPR experiments show loss of the characteristic iron-nitrosyl S = 1/2 signal and development of another S = 1/2 signal, which is characteristic of a type 2 Cu2+ species (g‖ = 2.40, g⊥ = 2.082, A‖ = 122 cm−1, A⊥ = 16 cm−1; Fig. 3B). Quantification of these EPR signals against a Cu2+ standard indicates that both 2-NO and 2-NO-O2 have one paramagnetic center. On the other hand, addition of 1 eq of O2− to 1-NO shifts the absorption bands from 425 nm, 548 nm to 428 nm, 538 nm (1-NO-S in Figs. 2A and 3A) and results in decay of the iron-nitrosyl S = 1/2 EPR signal. No other new EPR signals are observed in 1-NO-S and 2-NO-O2 even at 4 K (Fig. S2), which suggests the absence of any high-spin ferric species. Thus, both the UV-Vis and EPR data of 1-NO-S and 2-NO-O2 are consistent with an FeII end product.
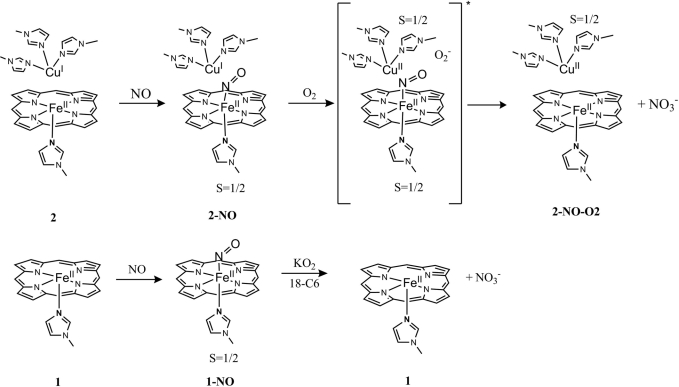
Scheme 1.
Proposed reaction mechanism of an NO-inhibited CcO model (2-NO) with O2 and Fe-only NO-bound ferrous porphyrin (1-NO) with O2−. The ligand superstructures are not included for clarity.
Fig. 2.
Absorption spectrum of the NO derivatives of 1 (A) and 2 (B) and their reactions with O2 and O2−.
Fig. 3.
EPR spectra of the NO derivatives of 1 (A) and 2 (B) and their reactions with O2 and O2−. Frequency, 9.38 MHz; power, 10 mW; gain, 5 × 103; modulation amplitude, 10 G; temperature, 77 K.
These results, summarized in Scheme 1, suggest that an iron-nitrosyl species lacking a distal CuI is inert toward oxygen but is reactive toward superoxide, forming a ferrous species (no intermediates were detected in this reaction even at −78°C, which is not unexpected, because peroxinitrite species, a very strong candidate for the intermediate, is very unstable and should rapidly isomerize in organic solvents). We propose that in the presence of distal CuI dioxygen is reduced in situ to O2−, which reacts with the iron-nitrosyl species, transforming it into the ferrous heme active site [the potential of the Cu site is ≈0 mV (18), which can reduce O2 to O2− (−100 mV) in presence of excess O2]. This O2−-dependent destruction of the stable ferrous nitrosyl species provides a plausible mechanism for the recovery of CcO from NO inhibition in the presence of both O2 and an electron from the CuB site. This reaction probably proceeds via a peroxynitrite intermediate, which forms by a reaction between superoxide and the NO complex. The peroxynitrite readily isomerizes into nitrate in organic solvents (25) (under physiological conditions, this peroxynitrite would be reduced to nitrite at the fully reduced active site of CcO).
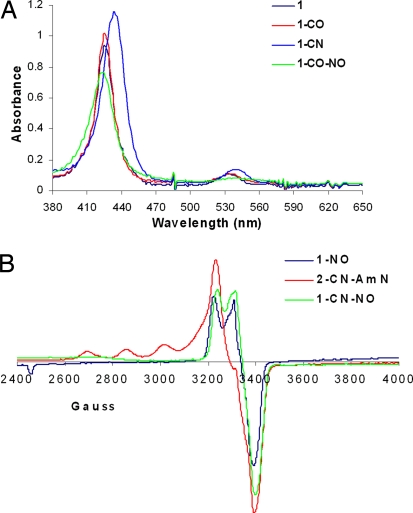
Adding CO to a solution of the unsubstituted ferrous-heme 1 leads to sharpening of the Soret and a small red shift of the band at 536 nm, which is indicative of the ferrous carbonyl complex 1-CO (λmax = 427 nm, 538 nm; Fig. 4A, red). Adding 10 eq of CN− to 1 produces a red shift in both the Soret and the Q band, suggesting formation of a CN− complex 1-CN (λmax = 433 nm, 541 nm; Fig. 4A, blue). Both of these molecules are either produced in the human body or present in our diet or environment, and they are known to be potent inhibitors of CcO. We have observed that both CO and CN− are replaced when equimolar NO (λmax = 425 nm, 548 nm; Fig. 4A, green) is added to a solution of 1-CN (Fig. 4A, blue) or 1-CO (Fig. 4A, red) (the substitution of CO by NO is much slower than that of CN−). This result is further supported by the EPR spectrum of 1-CN-NO, which shows the characteristic S = 1/2 iron-nitrosyl signal ≈3,200–3,600 G (Fig. 3A) after adding NO to the diamagnetic 1-CN species (Fig. 4B, green). Parallel experiments were also performed with the active site of myoglobin. The absorption spectrum of Mb-CO has a Soret at 423 nm and Q bands at 544 and 582 nm that are shifted to 424, 551, and 582 nm, respectively, after adding NO, indicating displacement of CO to form the NO complex (26, 27).
Fig. 4.
Absorption (A) and EPR (B) spectra of CO and CN derivatives of 1 and 2 and their reactions with NO and AmN, respectively.
AmN is commonly used as an immediate treatment for accidental exposure to CN. When AmN is added to 2-CN (prepared by adding 10 eq of CN− to 2), an EPR spectrum is obtained (2-CN-AmN; Fig. 4B, red), which suggests a mixture of an oxidized Cu2+ (indicated by the four-line hyperfine) and S = 1/2 iron-nitrosyl species (indicated by the feature at 3,400 G). The total spin integration against a Cu2+ standard shows the presence of two paramagnetic species in 2-CN-AmN. In contrast, no reaction was observed between AmN and copper free 1. We propose that AmN is reduced to amyl alkoxide and NO by the reduced CuB, which becomes oxidized to Cu2+ (Scheme 2). This NO generated in situ from AmN should replace the CN−, forming an iron-nitrosyl species at the heme a3 site. This hypothesis was also evaluated by adding AmN to 2-CO (Fig. S3).
Scheme 2.
Proposed mechanism for recovery from inhibition via in situ NO generation from AmN. The ligand superstructure is not included for clarity.
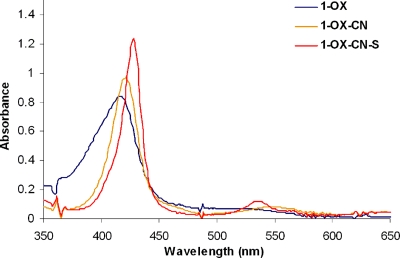
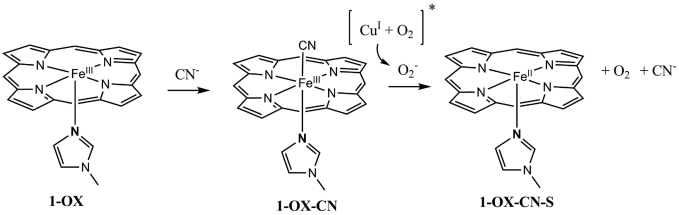
CN− is known to bind the oxidized FeIII form with a much higher affinity than to FeII. In CcO, CN− binding shifts the reduction potential of the heme a3 site by −200 mV (i.e., from +350 to +150 mV against a normal hydrogen electrode). At this potential the heme a3 site could not be reduced by heme a, which has a reduction potential of only +250 mV and is unchanged by CN− binding to heme a3 (19). This −200 mV decrease in reduction potential of heme a3 should inhibit turnover of CcO. Binding 1 eq of CN− to the oxidized model 1-OX (λmax = 415 nm, 519 nm; Fig. 5) in dichloromethane red shifts the UV-Vis (1-OX-CN, λmax = 423 nm, 555 nm; Fig. 5). Although this CN− complex is stable in O2, it is readily reduced to the ferrous form by addition of 1 eq of O2− (1-OX-CN-S, λmax = 428 nm, 538 nm; Fig. 5 red). Scheme 3 represents a likely mechanism that accounts for these events. In CcO the O2− could be produced in situ after the 1-electron reduction of O2 by the distal CuI as suggested by the reaction of 2-NO with O2 (see above).
Fig. 5.
UV-Vis spectrum of CN− derivative of 1-OX and its subsequent reaction with O2−.
Scheme 3.
Proposed mechanism for recovery from CN− inhibition of ferric CcO. The ligand superstructure is not included for clarity.
In summary our results strongly support the hypothesis by Pearce et al. (16) regarding the mechanism for recovery from inhibition of CcO by NO. NO reacts with our heme a3 model to form an iron-nitrosyl complex that is stable in O2. However, in the presence of a reduced CuB in the distal site, O2 generates O2−, which reacts with the iron-nitrosyl complex, possibly via a peroxynitrite intermediate, regenerating a ferrous heme a3 site ready for turnover. Given the high binding constant of NO with heme a3 and generation of NO by NOS adjacent to the mitochondria, we propose that this reaction recurs repeatedly during the normal turnover of CcO and plays a regulatory role in the functioning of CcO. NO also may be an active component in the defense of CcO against inhibitors such as CN− and CO. Because of its extremely high binding affinity for reduced heme a3, NO can replace either of these ligands, forming a stable ferrous nitrosyl complex, which could then be oxidized by O2− generated in situ, thereby regenerating the active enzyme. This in situ generation of O2− should provide also a defense against inhibitors that target the oxidized heme a3 site (anionic ligands such as CN−, N3−, etc.) by reducing the oxidized site to the corresponding ferrous forms, which have much higher NO affinities. Indeed, an increase of O2− concentration has been observed in CcO during turnover when the heme a3 site is inhibited by ligand binding (28). Thus, a symbiotic interaction between a potent inhibitor (NO) and a reactive oxygen species by-product (O2−) should protect CcO from external inhibitors. While NO defends the reduced site, O2− defends the oxidized site.
Materials and Methods
All chemical manipulations were carried out under N2 atmosphere unless mentioned otherwise. The synthesis of 1 and 2 has been reported previously (29). These are generally synthesized as the FeII and FeII/CuI forms, which are oxidized with stoichiometric amounts of ferrocenium tetrafluoroborate as and when mentioned. KO2 and [18]crown6 were purchased from Aldrich Chemicals and used without further purification. High-purity NO was purchased from Praxair and was further purified by bubbling it twice through 4 M KOH and H2O under N2 atmosphere. The solubility of NO in acetonitrile was taken to be 14 mM as reported (30). AmN was purchased from Aldrich and passed through a column of basic alumina before used to get rid of any amyl alcohol and nitrogen dioxide impurity that may have been present.
For the NO + O2 reactions, 1 eq of NO was added, and the formation of the nitrosyl species was confirmed by UV-Vis and EPR spectroscopy. To the same sample, excess O2 gas or 1 eq of superoxide was added and monitored by UV-Vis and EPR spectroscopy.
The CO samples were prepared by the addition to CO gas to the reduced complexes in a cuvette or EPR tube. The formation of the CO complexes was confirmed by UV-Vis spectroscopy. NO (soln) or AmN was then added to them.
CN− complexes were prepared by adding N,N,N,N-tetrabutylammonium cyanide to reduced or oxidized samples. The formation of the CN complexes was confirmed by UV-Vis and IR spectroscopy. NO (soln) or AmN was then added to them.
EPR spectra were obtained by using a Bruker EMX spectrometer, ER 041 XG microwave bridge, and ER 4102ST cavity. All X-band EPR samples were run at 77 K in a liquid nitrogen finger Dewar. A Cu standard (1.0 mM CuSO4·5H2O with 2 mM HCl and 2 M NaClO4) was used for spin quantitation of the EPR spectra. EPR data of the ferrous end product was obtained at liquid He temperature (4 K).
The UV-Vis spectra were collected by using a Hewlett–Packard apparatus 8452 glass cuvette (3-ml capacity) sealed with a 14/24 septum (solvent: dimethylformamide/dichloromethane; concentration in porphyrin: 1 × 10−5 M).
Supplementary Material
Acknowledgments.
We thank Somdatta Ghosh for help with the acquisition of low-temperature EPR data. This work was funded by National Institutes of Health Grant 5R01 GM-17880-35. R.A.D. is thankful for a Lavoisier Fellowship.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804257105/DCSupplemental.
References
- 1.Ferguson-Miller S, Babcock GT. Heme/copper terminal oxidases. Chem Rev. 1996;96:2889–2907. doi: 10.1021/cr950051s. [DOI] [PubMed] [Google Scholar]
- 2.Iwata S, Ostermeier C, Ludwig B, Michel H. Structure at 2.8 Å resolution of cytochrome c oxidase from Paracoccus denitrificans. Nature. 1995;376:660–669. doi: 10.1038/376660a0. [DOI] [PubMed] [Google Scholar]
- 3.Yoshikawa S, et al. Redox-coupled crystal structural changes in bovine heart cytochrome c oxidase. Science. 1998;280:1723–1729. doi: 10.1126/science.280.5370.1723. [DOI] [PubMed] [Google Scholar]
- 4.Petersen L. The effect of inhibitors on the oxygen kinetics of cytochrome c oxidase. Biochim Biophys Acta. 1977;460:299–307. doi: 10.1016/0005-2728(77)90216-x. [DOI] [PubMed] [Google Scholar]
- 5.Ghafourifar P, Richter C. Nitric oxide synthase activity in mitochondria. FEBS Lett. 1997;418:291–296. doi: 10.1016/s0014-5793(97)01397-5. [DOI] [PubMed] [Google Scholar]
- 6.Giulivi C, Poderoso JJ, Boveris A. Production of nitric oxide by mitochondria. J Biol Chem. 1998;273:11038–11043. doi: 10.1074/jbc.273.18.11038. [DOI] [PubMed] [Google Scholar]
- 7.Aregheore EM, Agunbiade OO. The toxic effects of cassava manihot esculenta grantz diets on humans: A review. Vet Hum Toxicol. 1991;33:274–275. [PubMed] [Google Scholar]
- 8.Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: Unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 9.Zuckerbraun BS, et al. Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. FASEB J. 2007;21:1099–1106. doi: 10.1096/fj.06-6644com. [DOI] [PubMed] [Google Scholar]
- 10.Ford PC, Lorkovic IM. Mechanistic aspects of the reactions of nitric oxide with transition-metal complexes. Chem Rev. 2002;102:993–1017. doi: 10.1021/cr0000271. [DOI] [PubMed] [Google Scholar]
- 11.Brunori M, et al. Nitric oxide and the respiratory enzyme. Biochim Biophys Acta. 2006;1757:1144–1154. doi: 10.1016/j.bbabio.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Cleeter MWJ, et al. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide: Implications for neurodegenerative diseases. FEBS Lett. 1994;345:50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 13.Moncada S, Erusalimsky JD. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat Rev Mol Cell Biol. 2002;3:214–220. doi: 10.1038/nrm762. [DOI] [PubMed] [Google Scholar]
- 14.Giuffre A, et al. On the mechanism of inhibition of cytochrome c oxidase by nitric oxide. J Biol Chem. 1996;271:33404–33408. doi: 10.1074/jbc.271.52.33404. [DOI] [PubMed] [Google Scholar]
- 15.Sarti P, et al. Nitric oxide and cytochrome c oxidase: Mechanisms of inhibition and NO degradation. Biochem Biophys Res Commun. 2000;274:183–187. doi: 10.1006/bbrc.2000.3117. [DOI] [PubMed] [Google Scholar]
- 16.Pearce LL, et al. The catabolic fate of nitric oxide: The nitric oxide oxidase and peroxynitrite reductase activities of cytochrome oxidase. J Biol Chem. 2002;277:13556–13562. doi: 10.1074/jbc.M109838200. [DOI] [PubMed] [Google Scholar]
- 17.Wainio WW, Greenlees J. Complexes of cytochrome c oxidase with cyanide and carbon monoxide. Arch Biochem Biophys. 1960;90:18–21. doi: 10.1016/0003-9861(60)90605-6. [DOI] [PubMed] [Google Scholar]
- 18.Vanbuuren KJH, Nicholls P, Van Gelder V. Biochemical and biophysical studies on cytochrome aa3: VI. Reaction of cyanide with oxidized and reduced enzyme. Biochim Biophys Acta. 1972;256:258–260. doi: 10.1016/0005-2728(72)90057-6. [DOI] [PubMed] [Google Scholar]
- 19.Kojima N, Palmer G. Further characterization of the potentiometric behavior of cytochrome oxidase cytochrome a stays low spin during oxidation and reduction. J Biol Chem. 1983;258:14908–14913. [PubMed] [Google Scholar]
- 20.Collman JP, Boulatov R, Sunderland CJ, Fu L. Functional analogues of cytochrome c oxidase, myoglobin, and hemoglobin. Chem Rev. 2004;104:561–588. doi: 10.1021/cr0206059. [DOI] [PubMed] [Google Scholar]
- 21.Collman JP, et al. A cytochrome c oxidase model catalyzes oxygen to water reduction under rate-limiting electron flux. Science. 2007;315:1565–1568. doi: 10.1126/science.1135844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collman JP, Decréau RA, Yan Y, Yoon J, Solomon EI. Intramolecular single-turnover reaction in a cytochrome c oxidase model bearing a Tyr244 mimic. J Am Chem Soc. 2007;129:5794–5795. doi: 10.1021/ja0690969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens TH, Bocian DF, Chan SI. EPR studies of 15NO-ferrocytochrome a3 in cytochrome c oxidase. FEBS Lett. 1979;97:314–316. doi: 10.1016/0014-5793(79)80110-6. [DOI] [PubMed] [Google Scholar]
- 24.Yoshimura T. 5-Coordinated and 6-coordinated nitrosyl iron(II) complexes of tetrakis (para-substituted phenyl) porphyrins: Substituent effect on the EPR parameters and the NO stretching frequencies. Bull Chem Soc Jpn. 1991;64:2819–2828. [Google Scholar]
- 25.Koppenol WH, Kissner R, Beckman JS. Syntheses of peroxynitrite: To go with the flow or on solid grounds? Methods Enzymol. 1996;269:296–302. doi: 10.1016/s0076-6879(96)69030-2. [DOI] [PubMed] [Google Scholar]
- 26.Yonetani T, et al. Electromagnetic properties of hemoproteins: V. Optical and electron-paramagnetic resonance characteristics of nitric-oxide derivatives of metalloporphyrin-apohemoprotein complexes. J Biol Chem. 1972;247:2447–2455. [PubMed] [Google Scholar]
- 27.Bowen WJ. The absorption spectra and extinction coefficients of myoglobin. J Biol Chem. 1949;179:235–245. [PubMed] [Google Scholar]
- 28.Sipos I, Tretter L, Adam-Vizi V. Quantitative relationship between inhibition of respiratory complexes and formation of reactive oxygen species in isolated nerve terminals. J Neurochem. 2003;84:112–118. doi: 10.1046/j.1471-4159.2003.01513.x. [DOI] [PubMed] [Google Scholar]
- 29.Collman JP, Sunderland CJ, Boulatov R. Biomimetic studies of terminal oxidases: Trisimidazole picket metalloporphyrins. Inorg Chem. 2002;41:2282–2291. doi: 10.1021/ic011191p. [DOI] [PubMed] [Google Scholar]
- 30.Shaw AW, Vosper AJ. Solubility of nitric oxide in aqueous and non-aqueous solvents. J Chem Soc Faraday Trans 1. 1977;73:1239–1244. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.