Abstract
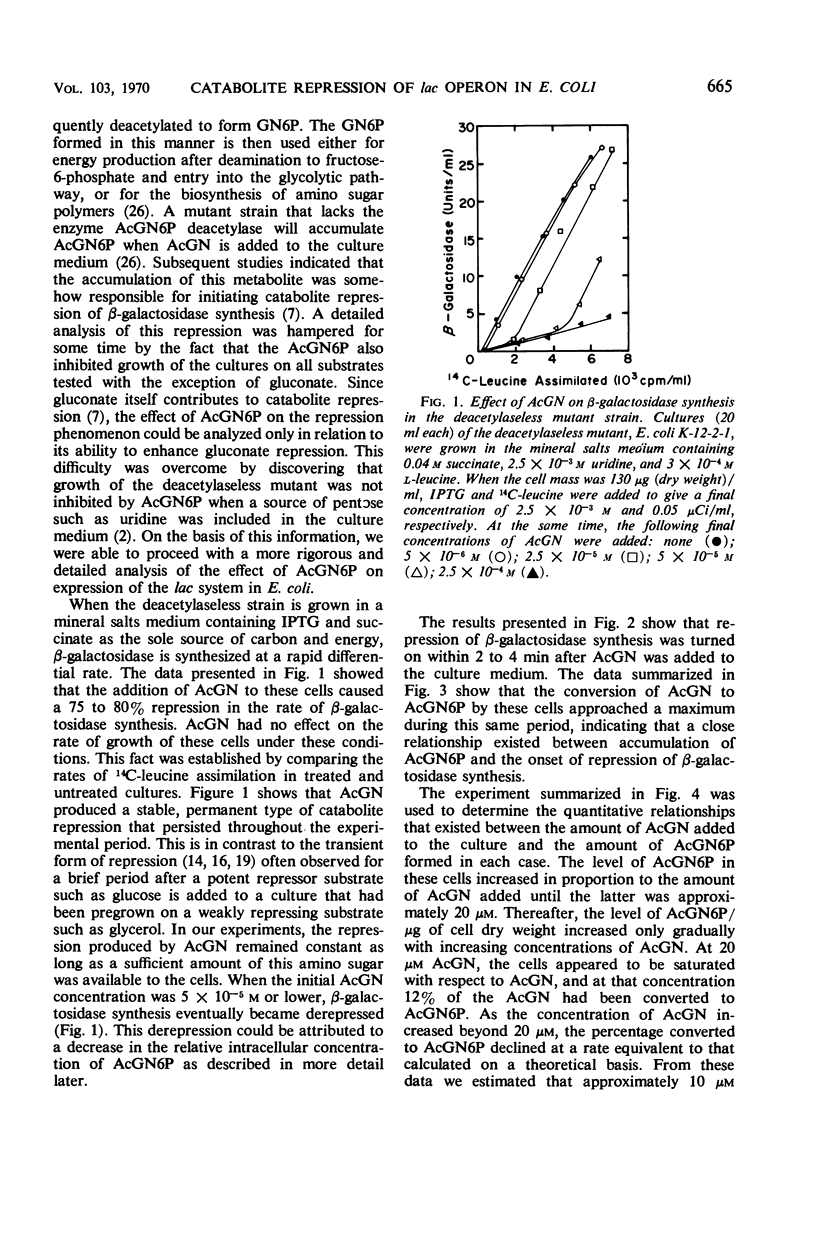
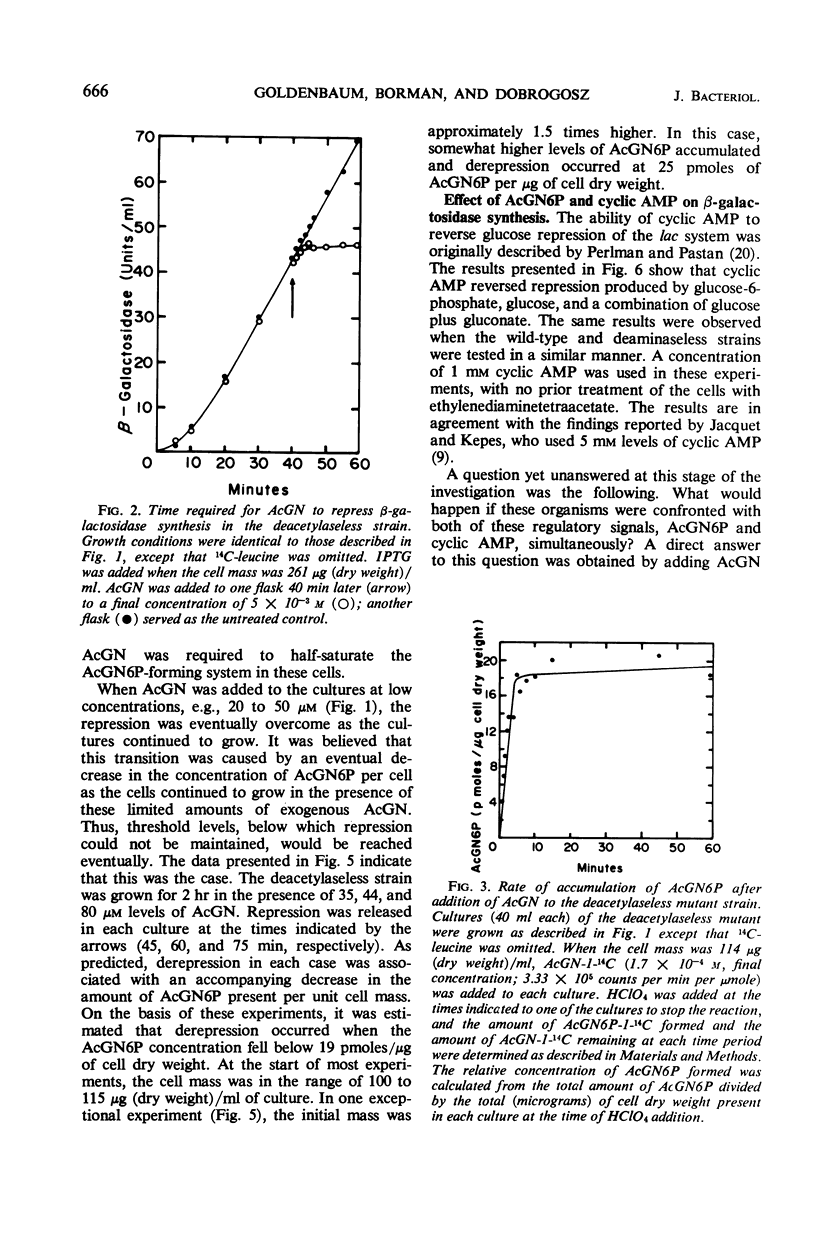
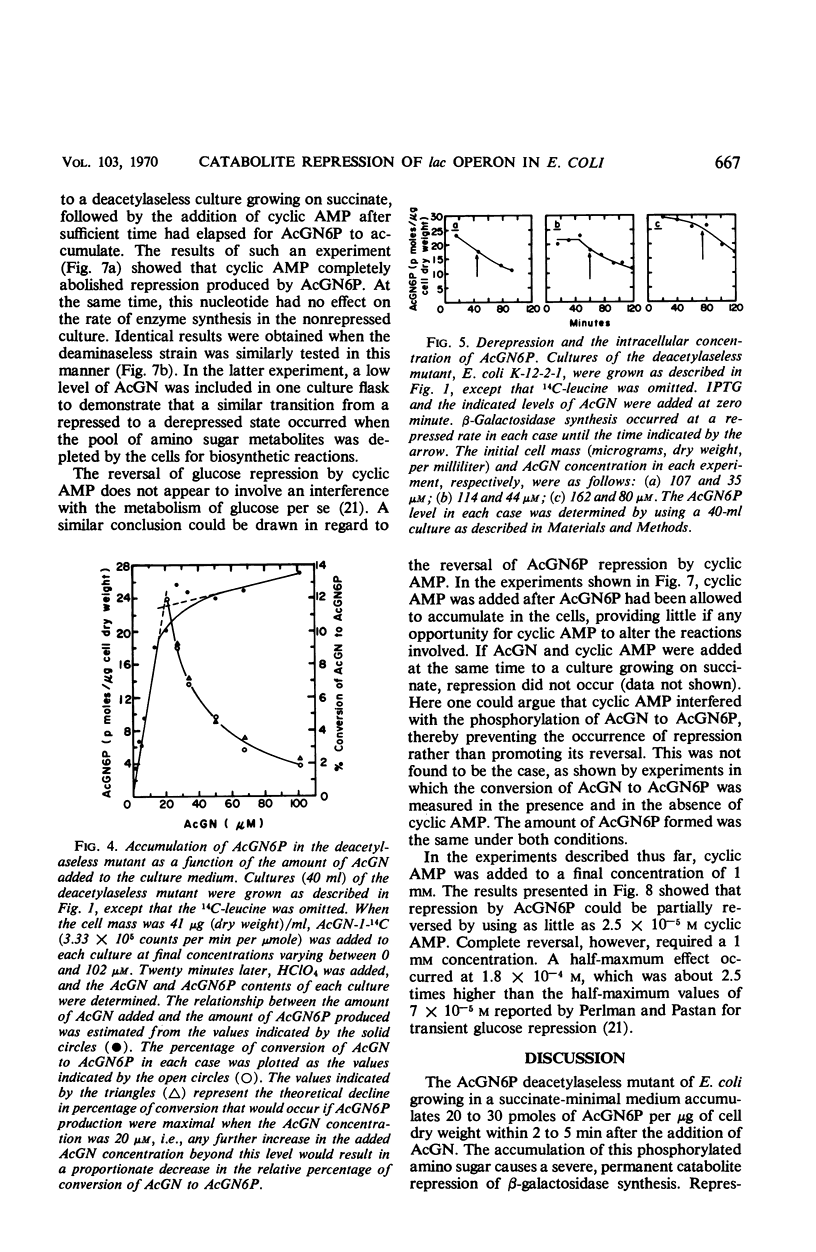
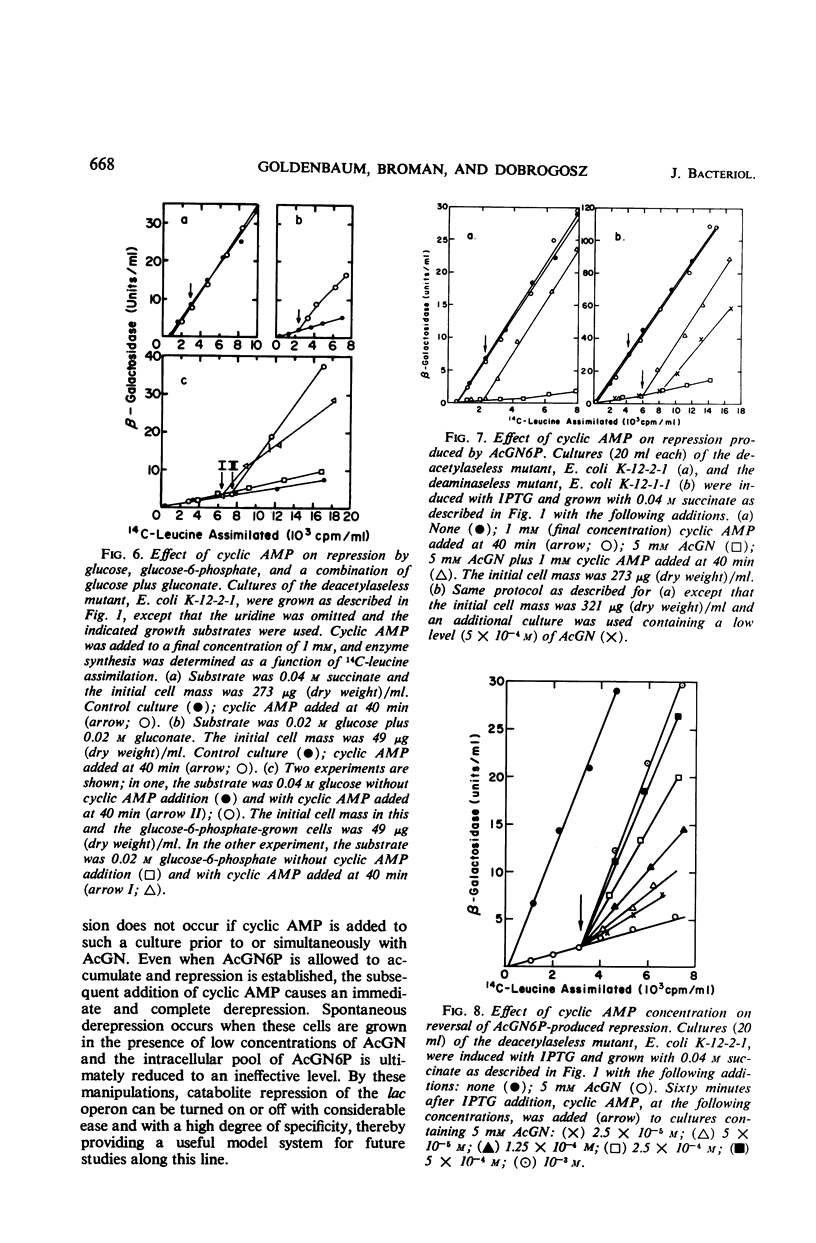
When an Escherichia coli mutant lacking the enzyme N-acetyl-glucosamine-6-phosphate (AcGN6P) deacetylase is grown in a succinate-mineral salts medium and exposed to an exogenous source of N-acetylglucosamine, approximately 20 to 30 pmoles of AcGN6P per μg of cell dry weight will accumulate in these cells. This accumulation occurs within 2 to 4 min after the addition of N-acetylglucosamine and is coincident with the production of a severe permanent catabolite repression of β-galactosidase synthesis. This repression does not occur if adenosine 3′,5′-cyclic phosphate (cyclic AMP) is added to the cells before AcGN6P accumulates. An immediate derepression occurs when cyclic AMP is added to cells that have already accumulated a large AcGN6P pool. These findings are consistent with the view that low-molecular-weight carbohydrate metabolites and cyclic AMP play key roles in the catabolite repression phenomenon, and that metabolites such as AcGN6P may participate in the represion mechanism by influencing either the formation or degradation of cyclic AMP in E. coli.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboud M., Burger M. The effect of catabolite repression and of cyclic 3',5' adenosine monophosphate on the translation of the lactose messenger RNA in Escherichia coli. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1023–1032. doi: 10.1016/0006-291x(70)90342-6. [DOI] [PubMed] [Google Scholar]
- Bernheim N. J., Dobrogosz W. J. Amino sugar sensitivity in Escherichia coli mutants unable to grow on N-acetylglucosamine. J Bacteriol. 1970 Feb;101(2):384–391. doi: 10.1128/jb.101.2.384-391.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broman R. L., Goldenbaum P. E., Dobrogosz W. J. The effect of amino acids on the ability of cyclic AMP to reverse catabolite repression in Escherichia coli. Biochem Biophys Res Commun. 1970 May 11;39(3):401–406. doi: 10.1016/0006-291x(70)90591-7. [DOI] [PubMed] [Google Scholar]
- Chambers D. A., Zubay G. The stimulatory effect of cyclic adenosine 3'5'-monophosphate on DNA-directed synthesis of beta-galactosidase in a cell-free system. Proc Natl Acad Sci U S A. 1969 May;63(1):118–122. doi: 10.1073/pnas.63.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISTLER J. J., MERRICK J. M., ROSEMAN S. Glucosamine metabolism. III. Preparation and N-acetylation of crystalline D-glucosamine- and D-galactosamine-6-phosphoric acids. J Biol Chem. 1958 Jan;230(1):497–509. [PubMed] [Google Scholar]
- De Crombrugghe B., Perlman R. L., Varmus H. E., Pastan I. Regulation of inducible enzyme synthesis in Escherichia coli by cyclic adenosine 3', 5'-monophosphate. J Biol Chem. 1969 Nov 10;244(21):5828–5835. [PubMed] [Google Scholar]
- Dobrogosz W. J. Corepressor system for catabolite repression of the lac operon in Escherichia coli. J Bacteriol. 1969 Mar;97(3):1083–1092. doi: 10.1128/jb.97.3.1083-1092.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Jacquet M., Kepes A. The step sensitive to catabolite repression and its reversal by 3'-5' cyclic AMP during induced synthesis of beta-galactosidase in E. coli. Biochem Biophys Res Commun. 1969 Jul 7;36(1):84–92. doi: 10.1016/0006-291x(69)90653-6. [DOI] [PubMed] [Google Scholar]
- LEVVY G. A., MCALLAN A. The N-acetylation and estimation of hexosamines. Biochem J. 1959 Sep;73:127–132. doi: 10.1042/bj0730127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- MAKMAN R. S., SUTHERLAND E. W. ADENOSINE 3',5'-PHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1965 Mar;240:1309–1314. [PubMed] [Google Scholar]
- Monard D., Janecek J., Rickenberg H. V. The enzymic degradation of 3',5' cyclic AMP in strains of E. Coli sensitive and resistant to catobolite repression. Biochem Biophys Res Commun. 1969 May 22;35(4):584–591. doi: 10.1016/0006-291x(69)90388-x. [DOI] [PubMed] [Google Scholar]
- Moses V., Prevost C. Catabolite repression of beta-galactosidase synthesis in Escherichia coli. Biochem J. 1966 Aug;100(2):336–353. doi: 10.1042/bj1000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okinaka R. T., Dobrogosz W. J. Catabolite repression and pyruvate metabolism in Escherichia coli. J Bacteriol. 1967 May;93(5):1644–1650. doi: 10.1128/jb.93.5.1644-1650.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paigen K. Phenomenon of transient repression in Escherichia coli. J Bacteriol. 1966 Mar;91(3):1201–1209. doi: 10.1128/jb.91.3.1201-1209.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Perlman R. L. Repression of beta-galactosidase synthesis by glucose in phosphotransferase mutants of Escherichia coli. Repression in the absence of glucose phosphorylation. J Biol Chem. 1969 Nov 10;244(21):5836–5842. [PubMed] [Google Scholar]
- Pastan I., Perlman R. L. The role of the lac promotor locus in the regulation of beta-galactosidase synthesis by cyclic 3',5'-adenosine monophosphate. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1336–1342. doi: 10.1073/pnas.61.4.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman R. L., De Crombrugghe B., Pastan I. Cyclic AMP regulates catabolite and transient repression in E. coli. Nature. 1969 Aug 23;223(5208):810–812. doi: 10.1038/223810a0. [DOI] [PubMed] [Google Scholar]
- Perlman R. L., Pastan I. Regulation of beta-galactosidase synthesis in Escherichia coli by cyclic adenosine 3',5'-monophosphate. J Biol Chem. 1968 Oct 25;243(20):5420–5427. [PubMed] [Google Scholar]
- Perlman R., Pastan I. Cyclic 3'5-AMP: stimulation of beta-galactosidase and tryptophanase induction in E. coli. Biochem Biophys Res Commun. 1968 Mar 27;30(6):656–664. doi: 10.1016/0006-291x(68)90563-9. [DOI] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- Tyler B., Loomis W. F., Jr, Magasanik B. Transient repression of the lac operon. J Bacteriol. 1967 Dec;94(6):2001–2011. doi: 10.1128/jb.94.6.2001-2011.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. J. Control of amino sugar metabolism in Escherichia coli and isolation of mutants unable to degrade amino sugars. Biochem J. 1968 Feb;106(4):847–858. doi: 10.1042/bj1060847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. J., Pasternak C. A. The purification and properties of N-acetylglucosamine 6-phosphate deacetylase from Escherichia coli. Biochem J. 1967 Oct;105(1):121–125. doi: 10.1042/bj1050121. [DOI] [PMC free article] [PubMed] [Google Scholar]


