Abstract
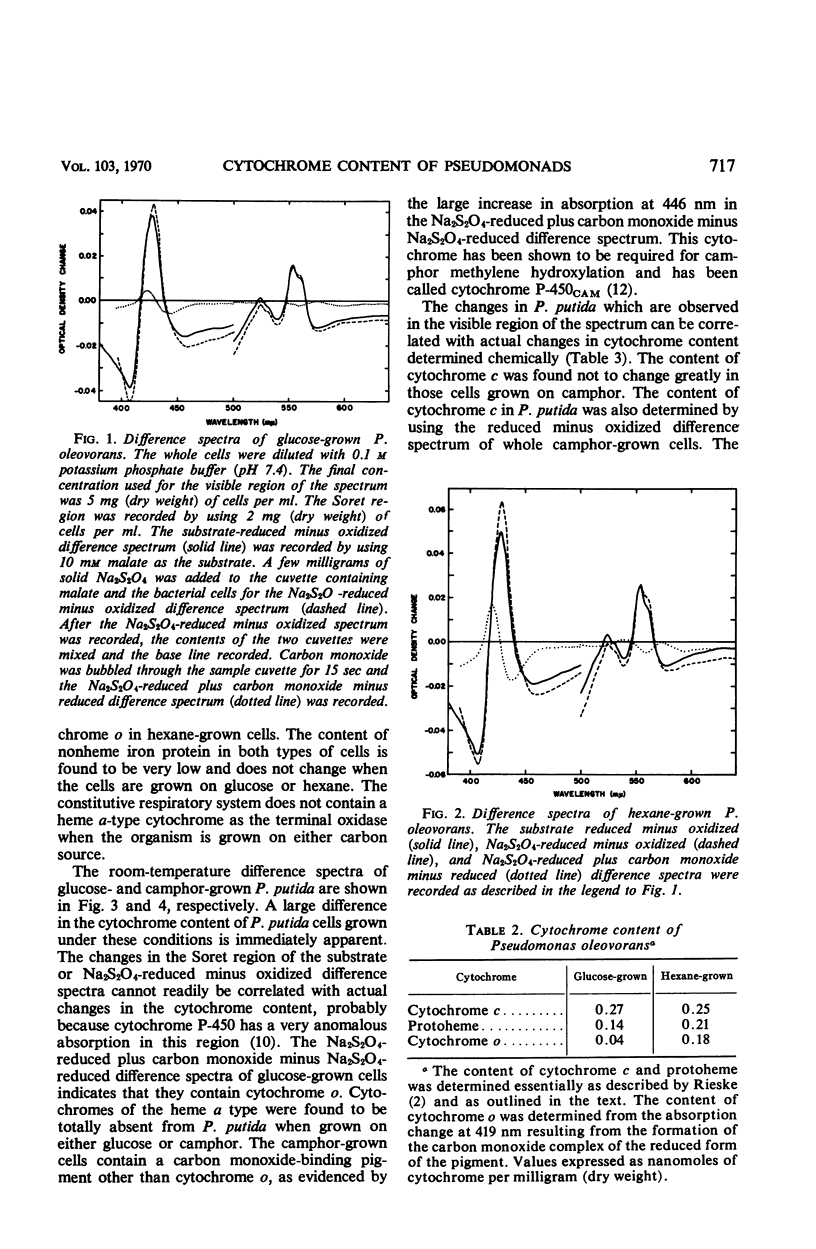
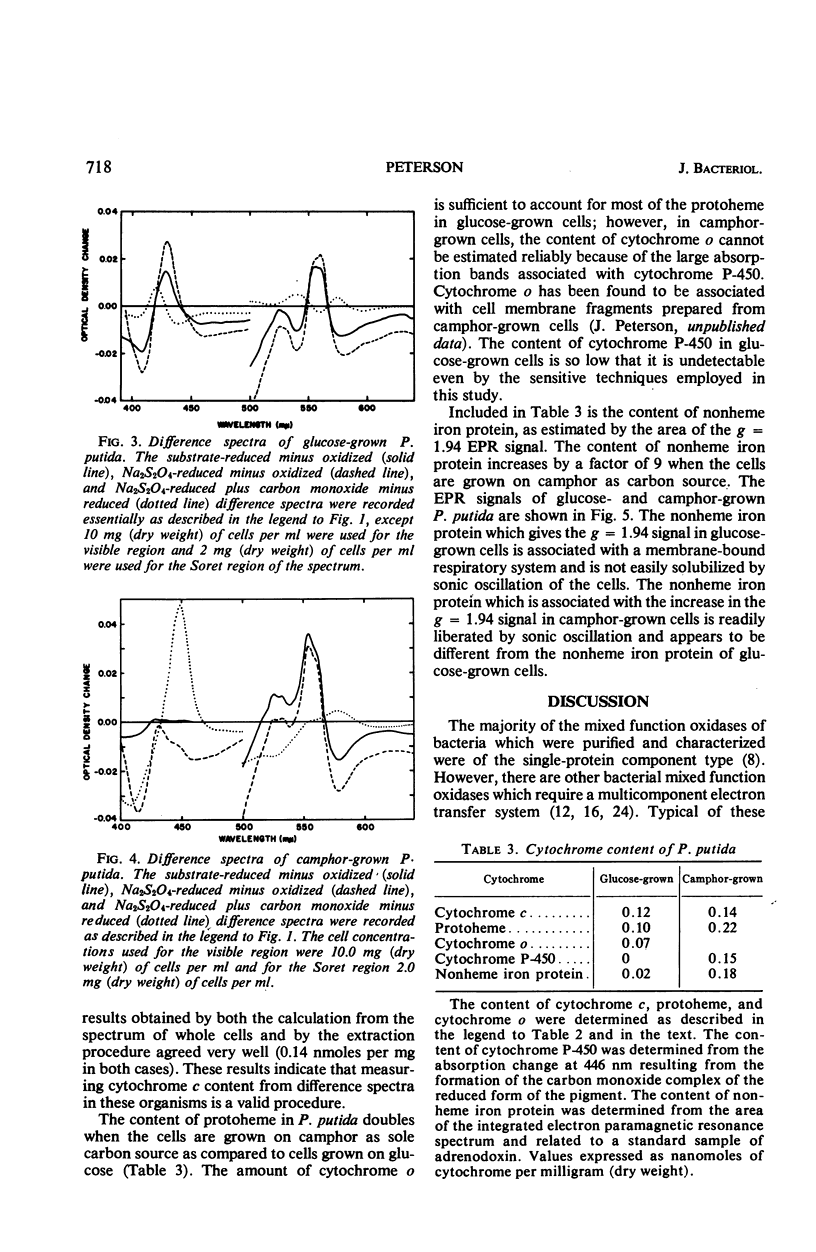
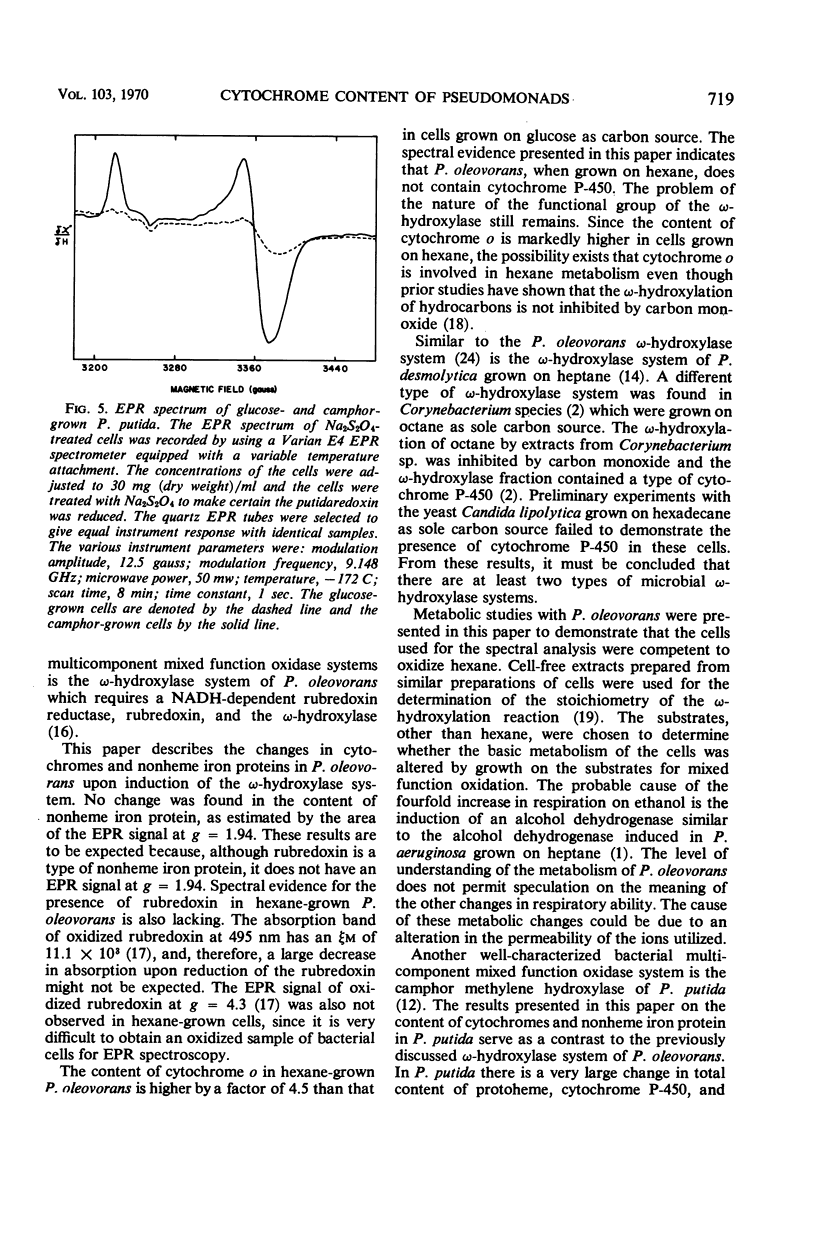
The cytochrome and nonheme iron protein content of two pseudomonads, Pseudomonas oleovorans and P. putida, containing mixed function oxidase systems was examined. The mixed function oxidase system of P. oleovorans and P. putida had previously been shown to be present in cells which had been grown on hexane and camphor, respectively, as energy source. The content of protoheme was found to increase significantly when the organisms were grown on the substrates for mixed function oxidation. The nonheme iron protein content increased significantly in the case of P. putida and was constant in P. oleovorans. The cytochrome c content was essentially constant in both pseudomonads. The content of cytochrome P-450 in P. putida increased from an immeasurably low amount to 0.15 nmoles per mg (dry weight). The content of cytochrome o in P. oleovorans increased by a factor of 4.5. P. oleovorans was not found to contain detectable quantities of cytochrome P-450 either in the presence or absence of the mixed function oxidase system.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AZOULAY E., HEYDEMAN M. T. Extraction and properties of alcohol dehydrogenase from Pseudomonas aeruginosa. Biochim Biophys Acta. 1963 May 7;73:1–6. doi: 10.1016/0006-3002(63)90353-6. [DOI] [PubMed] [Google Scholar]
- CASTOR L. N., CHANCE B. Photochemical determinations of the oxidases of bacteria. J Biol Chem. 1959 Jun;234(6):1587–1592. [PubMed] [Google Scholar]
- Cardini G., Jurtshuk P. Cytochrome P-450 involvement in the oxidation of n-octane b cell-free extracts of Corynebacterium sp. strain 7E1C. J Biol Chem. 1968 Nov 25;243(22):6070–6072. [PubMed] [Google Scholar]
- Cooper D. Y., Schleyer H., Rosenthal O. Role of cytochrome p-450 in mixed function oxidases using the reconstituted steroid 11beta-hydroxylase of adrenal mitochondria as an example. Hoppe Seylers Z Physiol Chem. 1968 Nov;349(11):1592–1598. [PubMed] [Google Scholar]
- Cushman D. W., Tsai R. L., Gunsalus I. C. The ferroprotein component of a methylene hydroxylase. Biochem Biophys Res Commun. 1967 Mar 9;26(5):577–583. doi: 10.1016/0006-291x(67)90104-0. [DOI] [PubMed] [Google Scholar]
- Gunsalus I. C. A soluble methylene hydroxylase system: structure and role of cytochrome P-450 and iron-sulfur protein components. Hoppe Seylers Z Physiol Chem. 1968 Nov;349(11):1610–1613. [PubMed] [Google Scholar]
- HASHIMOTO Y., YAMANO T., MASON H. S. An electron spin resonance study of microsomal electron transport. J Biol Chem. 1962 Dec;237:3843–3844. [PubMed] [Google Scholar]
- Hayaishi O. Enzymic hydroxylation. Annu Rev Biochem. 1969;38:21–44. doi: 10.1146/annurev.bi.38.070169.000321. [DOI] [PubMed] [Google Scholar]
- Hildebrandt A., Remmer H., Estabrook R. W. Cytochrome P-450 of liver microsomes--one pigment or many. Biochem Biophys Res Commun. 1968 Mar 27;30(6):607–612. doi: 10.1016/0006-291x(68)90555-x. [DOI] [PubMed] [Google Scholar]
- Imai K., Asano A., Sato R. Oxidative phosphorylation in Micrococcus denitrificans. I. Preparation and properties of phosphorylating membrane fragments. Biochim Biophys Acta. 1967;143(3):462–476. doi: 10.1016/0005-2728(67)90052-7. [DOI] [PubMed] [Google Scholar]
- KUSUNOSE M., KUSUNOSE E., COON M. J. ENZYMATIC OMEGA-OXIDATION OF FATTY ACIDS. I. PRODUCTS OF OCTANOATE, DECONATE, AND LAURATE OXIDATION. J Biol Chem. 1964 May;239:1374–1380. [PubMed] [Google Scholar]
- Katagiri M., Ganguli B. N., Gunsalus I. C. A soluble cytochrome P-450 functional in methylene hydroxylation. J Biol Chem. 1968 Jun 25;243(12):3543–3546. [PubMed] [Google Scholar]
- Kusunose M., Matsumoto J., Ichihara K., Kusunose E., Nozaka J. Requirement of three proteins for hydrocarbon oxidation. J Biochem. 1967 May;61(5):665–667. doi: 10.1093/oxfordjournals.jbchem.a128600. [DOI] [PubMed] [Google Scholar]
- Omura T., Sato R., Cooper D. Y., Rosenthal O., Estabrook R. W. Function of cytochrome P-450 of microsomes. Fed Proc. 1965 Sep-Oct;24(5):1181–1189. [PubMed] [Google Scholar]
- Peterson J. A., Basu D., Coon M. J. Enzymatic omega-oxidation. I. Electon carriers in fatty acid and hydrocarbon hydroxylation. J Biol Chem. 1966 Nov 10;241(21):5162–5164. [PubMed] [Google Scholar]
- Peterson J. A., Coon M. J. Enzymatic omega-oxidation. 3. Purification and properties of rubredoxin, a component of the omega-hydroxylation system of Pseudomonas oleovorans. J Biol Chem. 1968 Jan 25;243(2):329–334. [PubMed] [Google Scholar]
- Peterson J. A., Kusunose M., Kusunose E., Coon M. J. Enzymatic omega-oxidation. II. Function of rubredoxin as the electron carrier in omega-hydroxylation. J Biol Chem. 1967 Oct 10;242(19):4334–4340. [PubMed] [Google Scholar]
- Peterson J. A., McKenna E. J., Estabrook D. W., Coon M. J. Enzymic omega-oxidation: stoichiometry of the omega-oxidation of fatty acids. Arch Biochem Biophys. 1969 Apr;131(1):245–252. doi: 10.1016/0003-9861(69)90128-3. [DOI] [PubMed] [Google Scholar]
- Scholes P. B., Smith L. Composition and properties of the membrane-bound respiratory chain system of Micrococcus denitrificans. Biochim Biophys Acta. 1968 Feb 12;153(2):363–375. doi: 10.1016/0005-2728(68)90081-9. [DOI] [PubMed] [Google Scholar]
- Webster D. A., Hackett D. P. The purification and properties of cytochrome o from Vitreoscilla. J Biol Chem. 1966 Jul 25;241(14):3308–3315. [PubMed] [Google Scholar]
- Yu C. A., Gunsalus I. C. Monoxygenases. VII. Camphor ketolactonase I and the role of three protein components. J Biol Chem. 1969 Nov 25;244(22):6149–6152. [PubMed] [Google Scholar]


