Abstract
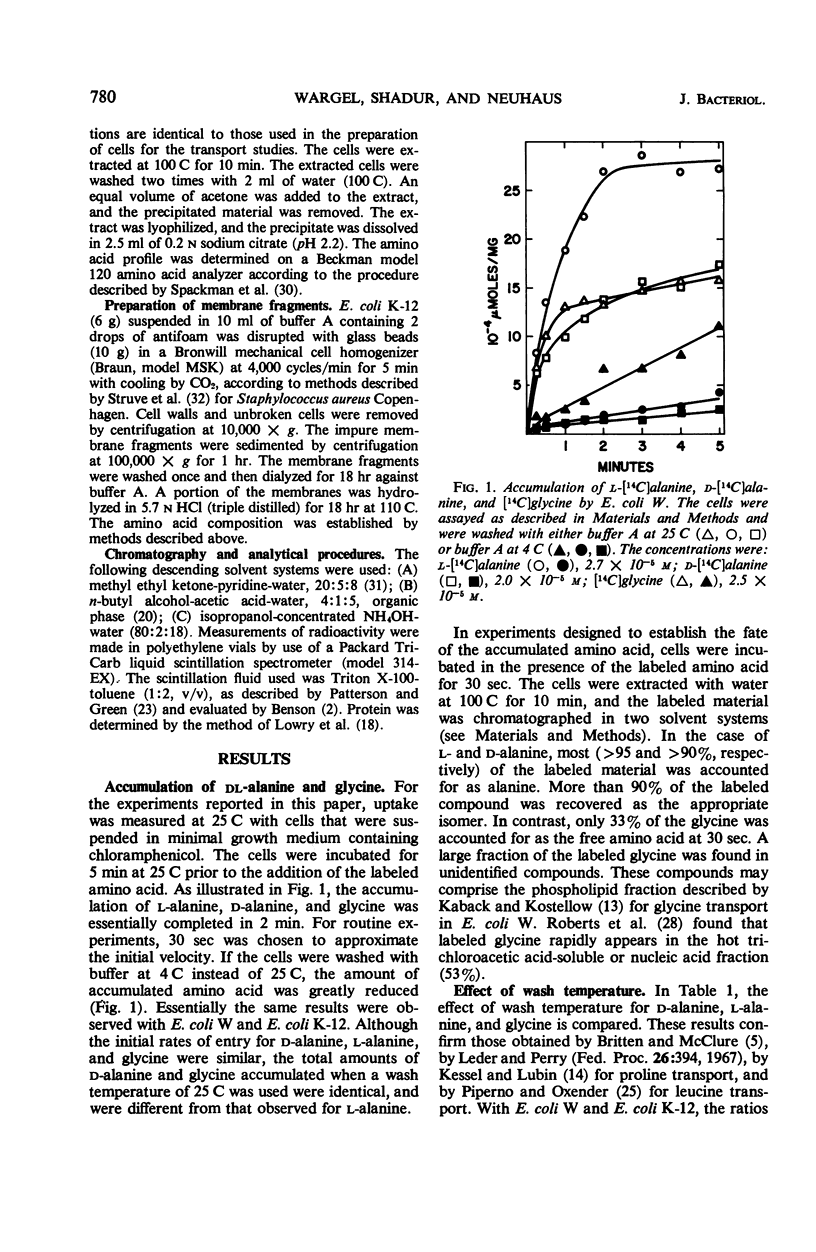
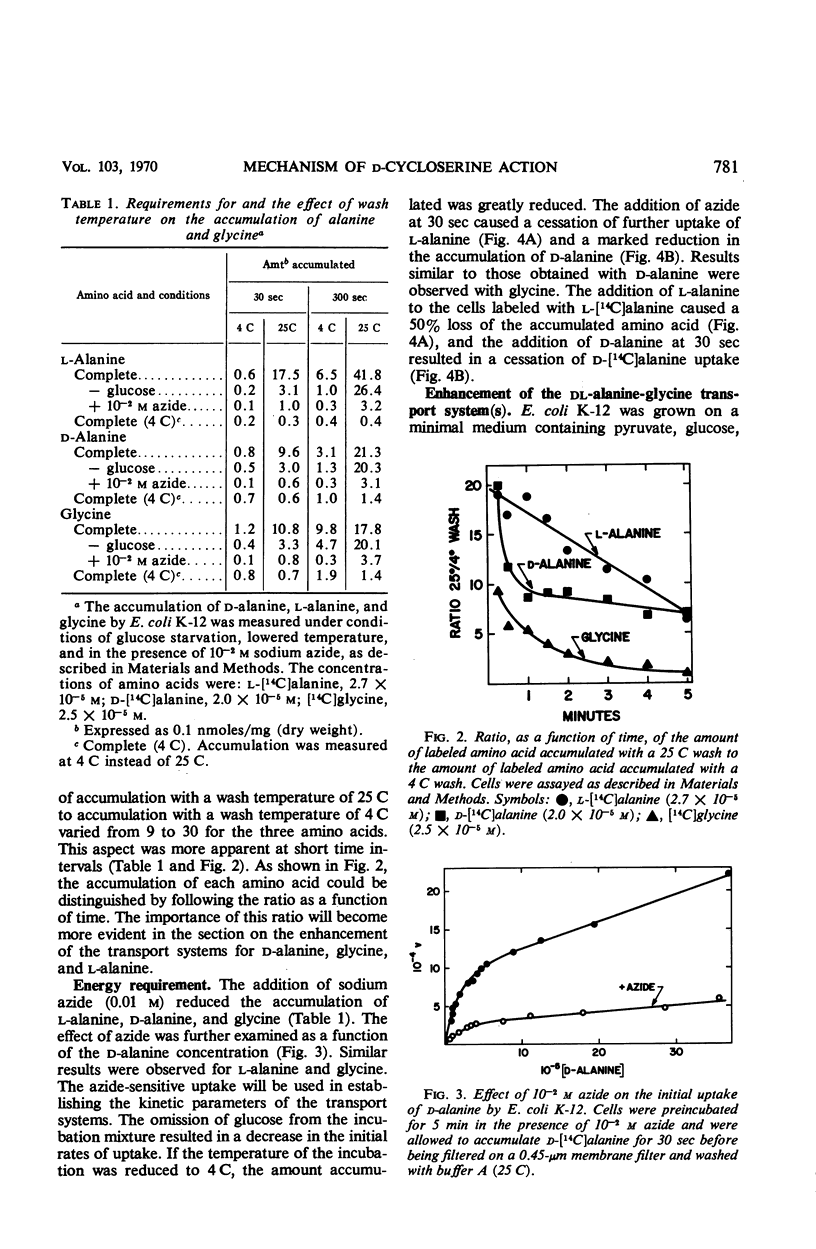
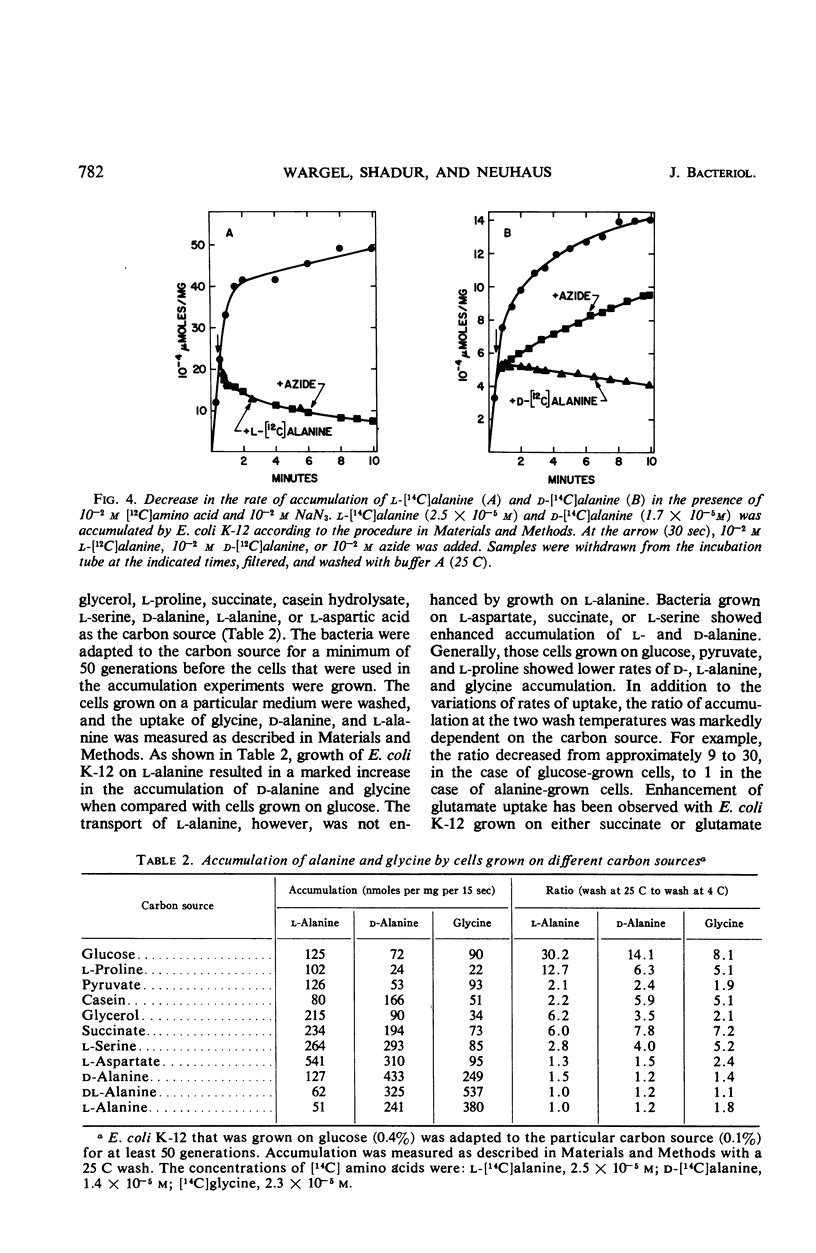
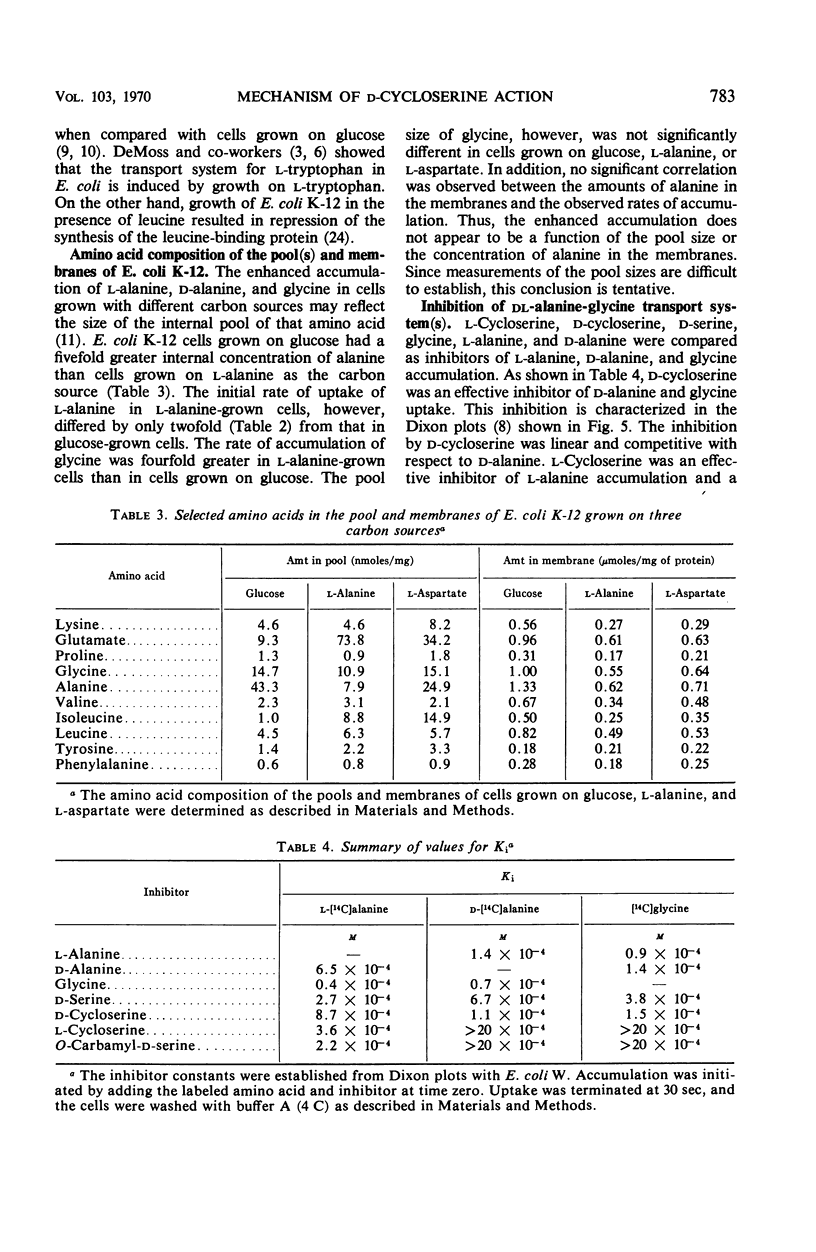
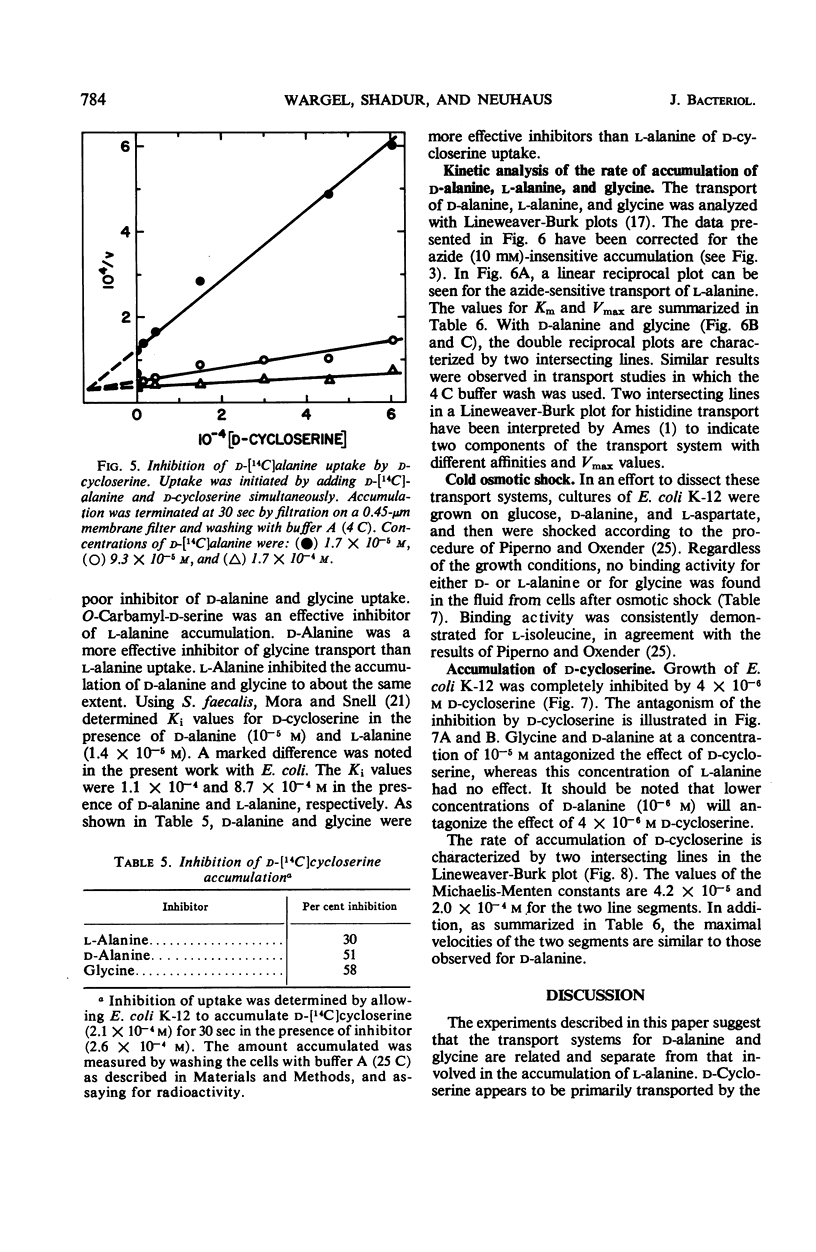
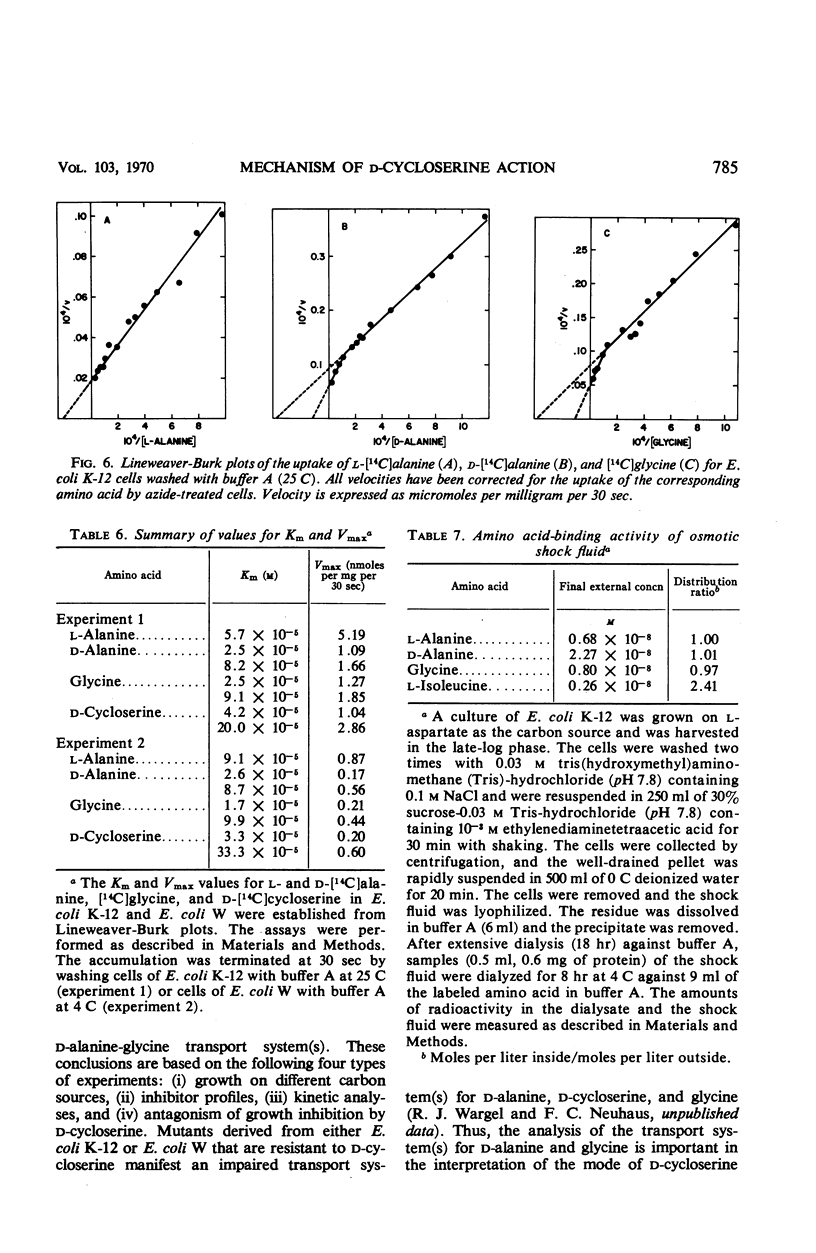
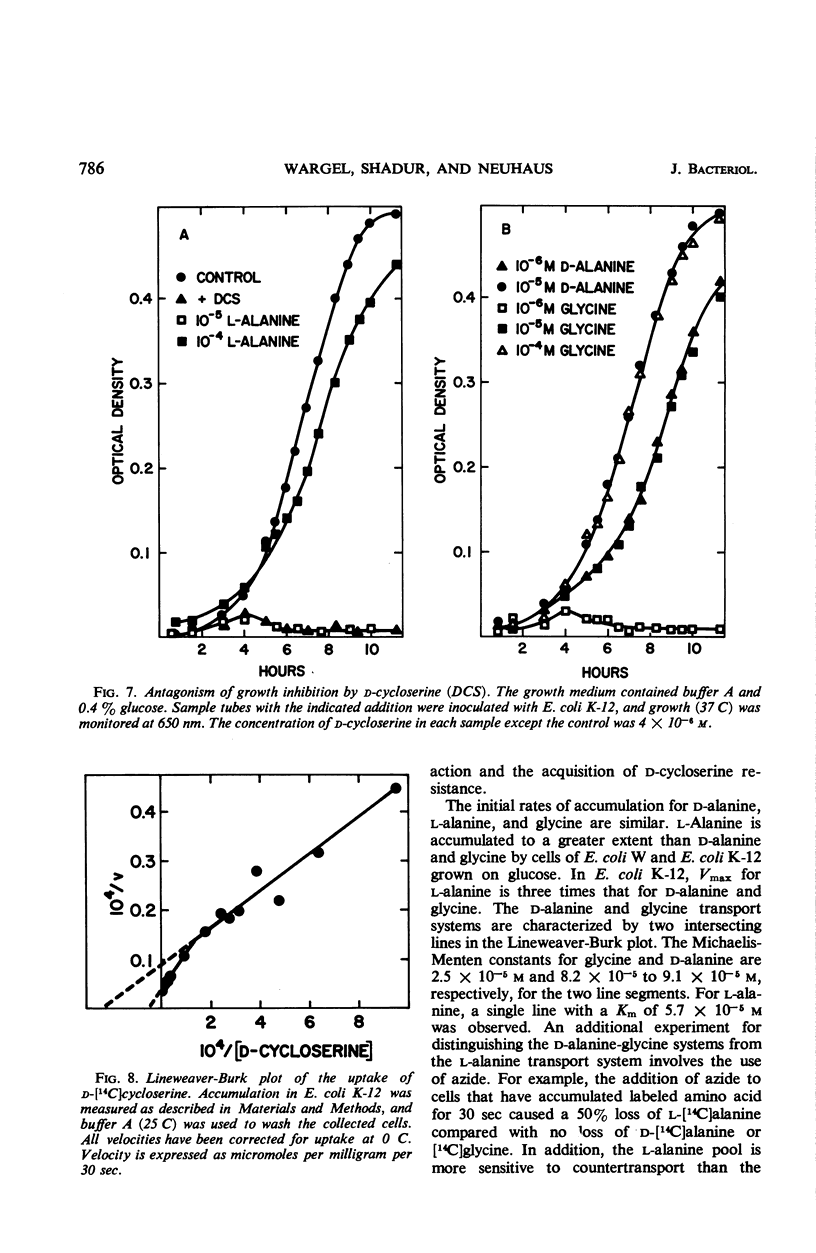
The accumulation of d-alanine, l-alanine, glycine, and d-cycloserine in Escherichia coli was found to be mediated by at least two transport systems. The systems for d-alanine and glycine are related, and are separate from that involved in the accumulation of l-alanine. d-Cycloserine appears to be primarily transported by the d-alanine-glycine system. The accumulation of d-alanine, glycine, and d-cycloserine was characterized by two line segments in the Lineweaver-Burk analysis, whereas the accumulation of l-alanine was characterized by a single line segment. d-Cycloserine was an effective inhibitor of glycine and d-alanine accumulation, and l-cycloserine was an effective inhibitor of l-alanine transport. The systems were further differentiated by effects of azide, enhancement under various growth conditions, and additional inhibitor studies. Since the primary access of d-cycloserine in E. coli is via the d-alanine-glycine system, glycine might be expected to be a better antagonist of d-cycloserine inhibition than l-alanine. Glycine and d-alanine at 10−5m antagonized the effect of d-cycloserine in E. coli, whereas this concentration of l-alanine had no effect.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES G. F. UPTAKE OF AMINO ACIDS BY SALMONELLA TYPHIMURIUM. Arch Biochem Biophys. 1964 Jan;104:1–18. doi: 10.1016/s0003-9861(64)80028-x. [DOI] [PubMed] [Google Scholar]
- BRITTEN R. J., McCLURE F. T. The amino acid pool in Escherichia coli. Bacteriol Rev. 1962 Sep;26:292–335. doi: 10.1128/br.26.3.292-335.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURROUS S. E., DEMOSS R. D. STUDIES ON TRYPTOPHAN PERMEASE IN ESCHERICHIA COLI. Biochim Biophys Acta. 1963 Aug 6;73:623–637. doi: 10.1016/0006-3002(63)90332-9. [DOI] [PubMed] [Google Scholar]
- Christensen H. N. Methods for distinguishing amino acid transport systems of a given cell or tissue. Fed Proc. 1966 May-Jun;25(3):850–853. [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern Y. S., Even-Shoshan A. Properties of the glutamate transport system in Escherichia coli. J Bacteriol. 1967 Mar;93(3):1009–1016. doi: 10.1128/jb.93.3.1009-1016.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern Y. S., Lupo M. Glutamate transport in wild-type and mutant strains of Escherichia coli. J Bacteriol. 1965 Nov;90(5):1288–1295. doi: 10.1128/jb.90.5.1288-1295.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESSEL D., LUBIN M. STABILITY OF ALPHA-HYDROGEN OF AMINO ACIDS DURING ACTIVE TRANSPORT. Biochemistry. 1965 Mar;4:561–565. doi: 10.1021/bi00879a029. [DOI] [PubMed] [Google Scholar]
- KESSEL D., LUBIN M. Transport of proline in Escherichia coli. Biochim Biophys Acta. 1962 Feb 12;57:32–43. doi: 10.1016/0006-3002(62)91074-0. [DOI] [PubMed] [Google Scholar]
- Kaback H. R., Kostellow A. B. Glycine uptake in Escherichia coli. I. Glycine uptake by whole cells of Escherichia coli W+ and a D-serine-resistant. J Biol Chem. 1968 Apr 10;243(7):1384–1389. [PubMed] [Google Scholar]
- LEACH F. R., SNELL E. E. The absorption of glycine and alanine and their peptides by Lactobacillus casei. J Biol Chem. 1960 Dec;235:3523–3531. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lynch J. L., Neuhaus F. C. On the mechanism of action of the antibiotic O-carbamyld-serine in Streptococcus faecalis. J Bacteriol. 1966 Jan;91(1):449–460. doi: 10.1128/jb.91.1.449-460.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Penrose W. R., Nichoalds G. E., Piperno J. R., Oxender D. L. Purification and properties of a leucine-binding protein from Escherichia coli. J Biol Chem. 1968 Nov 25;243(22):5921–5928. [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino acid transport systems in Escherichia coli K-12. J Biol Chem. 1968 Nov 25;243(22):5914–5920. [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino-acid-binding protein released from Escherichia coli by osmotic shock. J Biol Chem. 1966 Dec 10;241(23):5732–5734. [PubMed] [Google Scholar]
- Reitz R. H., Slade H. D., Neuhaus F. C. The biochemical mechanisms of resistance by streptococci to the antibiotics D-cycloserine and O-carbamyl-D-serine. Biochemistry. 1967 Aug;6(8):2561–2570. doi: 10.1021/bi00860a038. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ J. H., MAAS W. K., SIMON E. J. An impaired concentrating mechanism for amino acids in mutants of Escherichia coli resistant to L-canavanine and D-serine. Biochim Biophys Acta. 1959 Apr;32:582–583. doi: 10.1016/0006-3002(59)90650-x. [DOI] [PubMed] [Google Scholar]
- STRUVE W. G., NEUHAUS F. C. EVIDENCE FOR AN INITIAL ACCEPTOR OF UDP-NAC-MURAMYL-PENTAPEPTIDE IN THE SYNTHESIS OF BACTERIAL MUCOPEPTIDE. Biochem Biophys Res Commun. 1965 Jan 4;18:6–12. doi: 10.1016/0006-291x(65)90873-9. [DOI] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Partial separation of two pools of arginine in Escherichia coli; preferential use of exogenous rather than endogenous arginine for the biosynthesis of 1,4-diaminobutane. J Biol Chem. 1969 Dec 10;244(23):6383–6387. [PubMed] [Google Scholar]


