Abstract
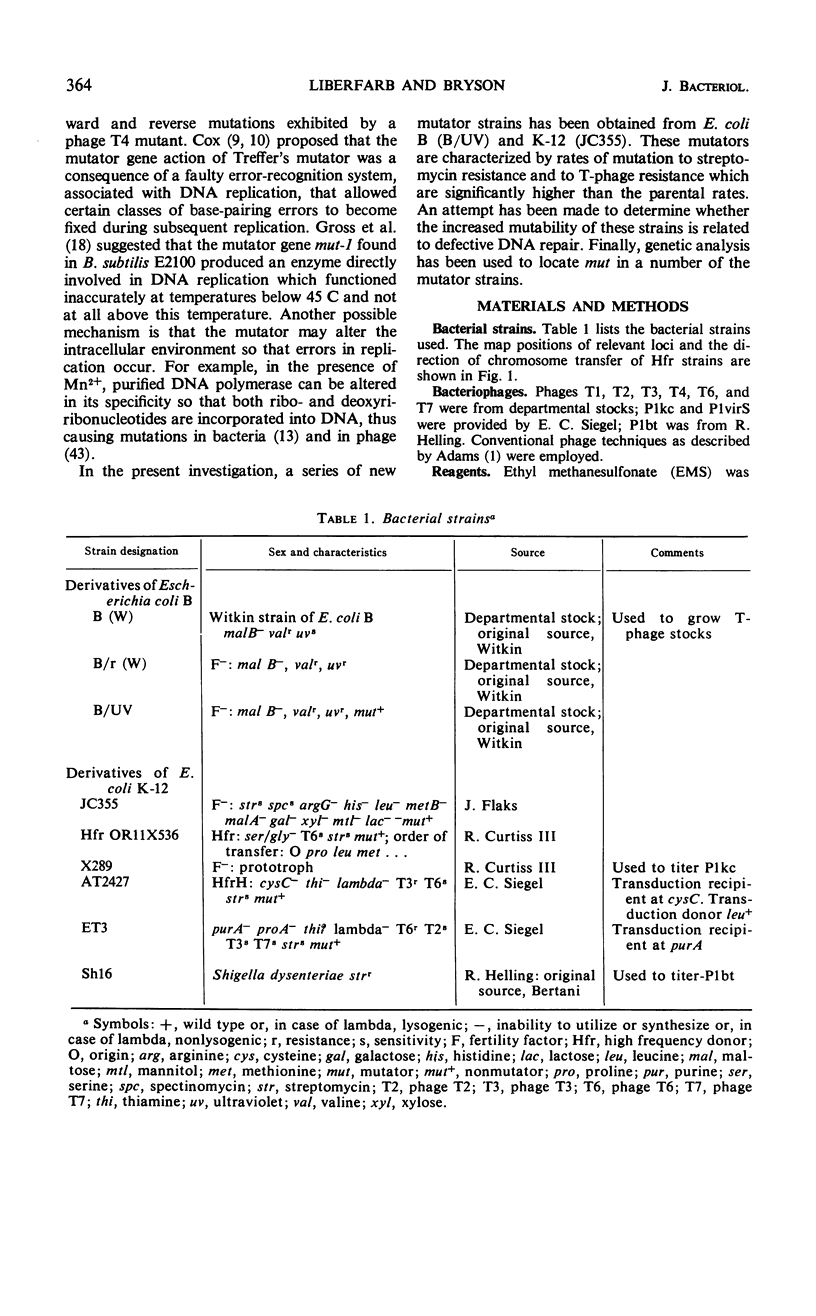
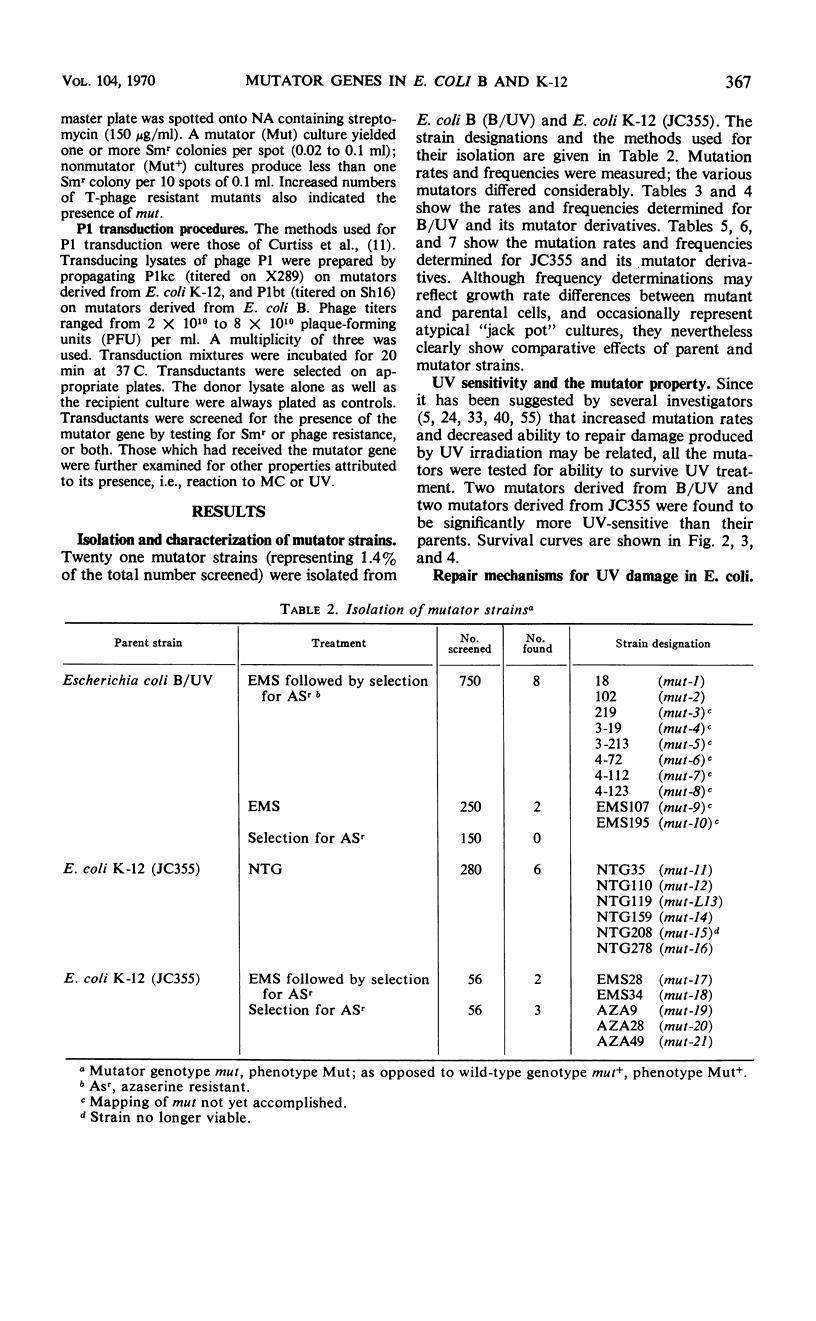
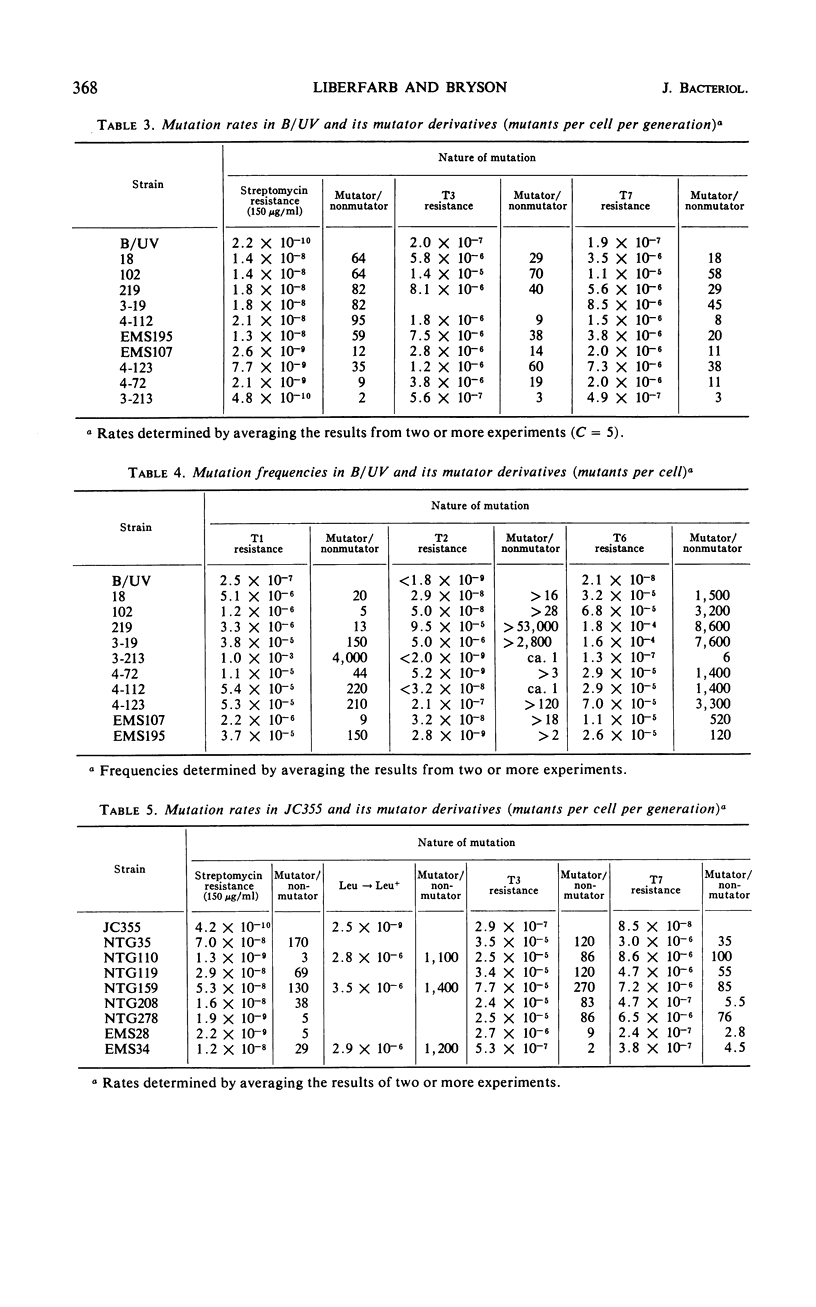
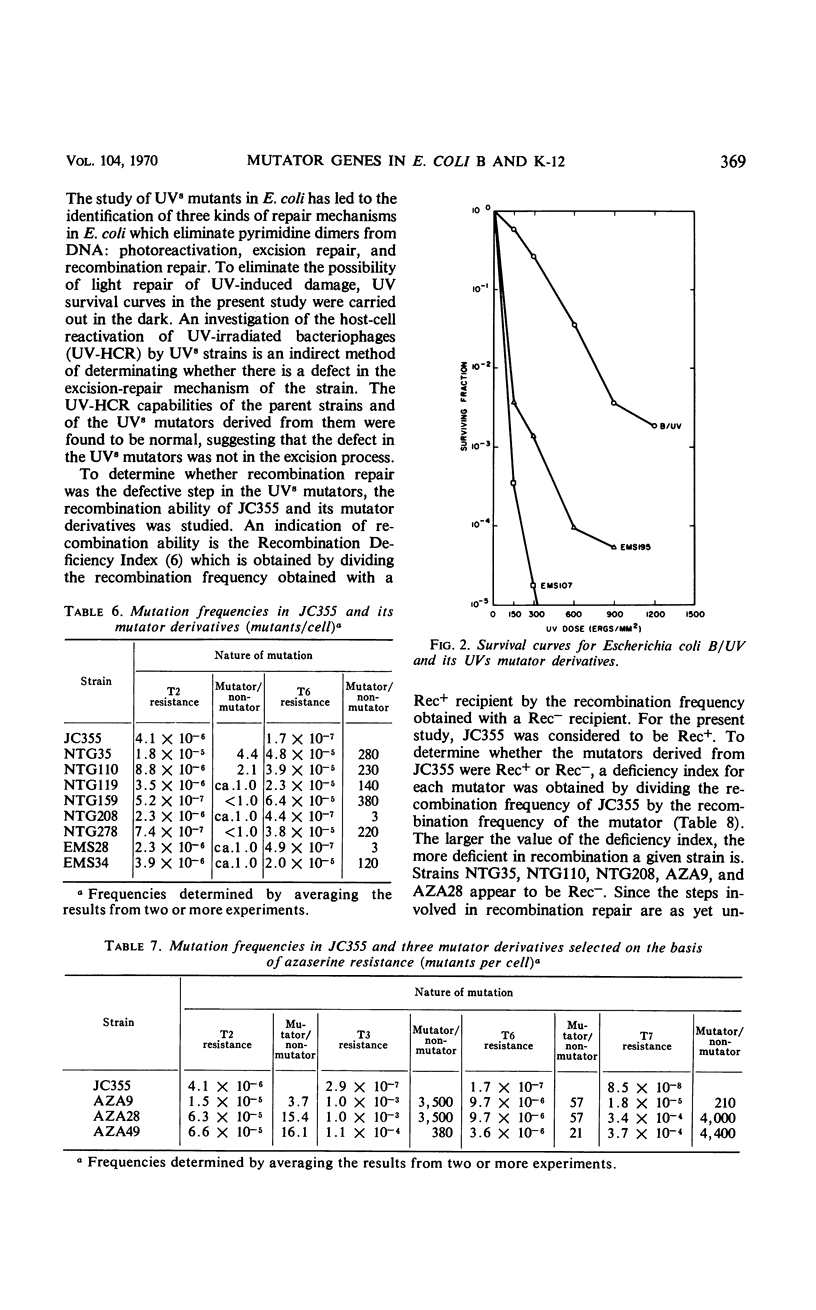
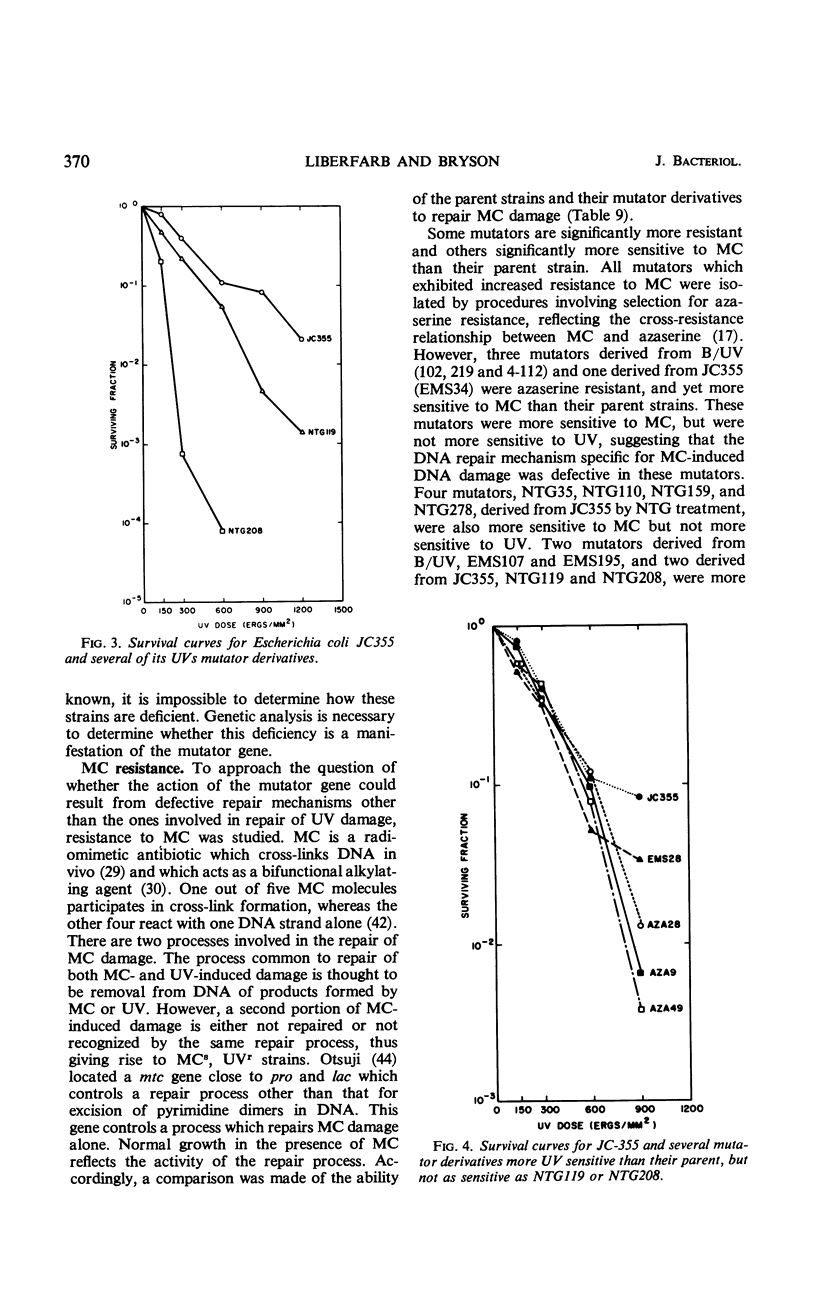
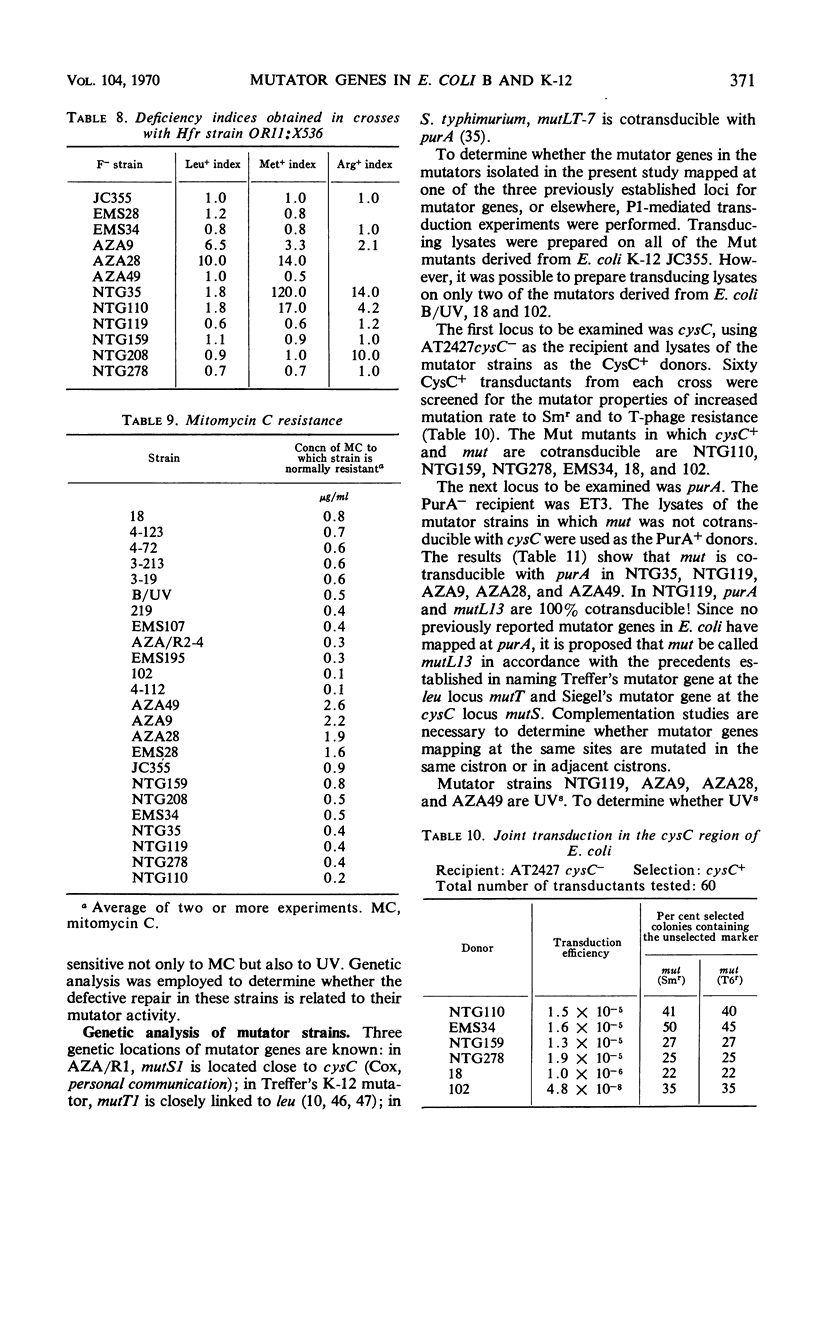
Twenty-one Mut mutants were obtained from Escherichia coli B (B/UV) and K-12 (JC355) after treatment with mutagens. These Mut strains are characterized by rates of mutation to streptomycin resistance and T-phase resistance which are significantly higher than the parental (Mut+) rates. Mutator genes in 12 strains have been mapped at three locations on the E. coli chromosome: one close to the leu locus; five close to the purA locus; and six close to cysC. In addition, eight mutator strains derived from E. coli B/UV are still unmapped. Some effort was made to deduce the mode of action of the mutator genes. These isolates have been examined for possible defects in deoxyribonucleic acid repair mechanisms (dark repair of ultraviolet damage, host-cell reactivation, recombination ability, repair of mitomycin C damage). By using transductional analysis, it was found that the ultraviolet sensitivity of NTG119 and its mutator property results from two separate but closely linked mutations. PurA+ transductants that receive mut from NTG119 or NTG35 are all more sensitive to mitomycin C than is the PurA recipient. Unless transduction selects for sensitivity, a probable interpretation is that defective repair of mitomycin C-induced damage is related to the mode of action of mut in these transductants and the donor. Abnormal purine synthesis may be involved in the mutability of some strains with cotransduction of the mutator properly and purA (100% cotransduction for NTG119). Three mutators are recombination-deficient and may have a defective step in recombination repair. One maps near three rec genes close to cysC.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., BURNS S. N. Genetic variation in the sex factor of Escherichia coli. J Bacteriol. 1960 Mar;79:321–330. doi: 10.1128/jb.79.3.321-330.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADELBERG E. A., COUGHLIN C. A. Bacterial mutation induced by thymine starvation. Nature. 1956 Sep 8;178(4532):531–532. doi: 10.1038/178531a0. [DOI] [PubMed] [Google Scholar]
- Berg C. M., Curtiss R., 3rd Transposition derivatives of an Hfr strain of Escherichia coli K-12. Genetics. 1967 Jul;56(3):503–525. doi: 10.1093/genetics/56.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhme H. Genetic instability of an ultraviolet-sensitive mutant of Proteus mirabilis. Biochem Biophys Res Commun. 1967 Jul 21;28(2):191–196. doi: 10.1016/0006-291x(67)90428-7. [DOI] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland J. C., Bryson V. Restriction in matings of Escherichia coli strain K-12 with strain B. Genetics. 1966 Aug;54(2):441–452. doi: 10.1093/genetics/54.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox E. C. Mutator gene action and the replication of bacteriophage lambda DNA. J Mol Biol. 1970 May 28;50(1):129–135. doi: 10.1016/0022-2836(70)90109-9. [DOI] [PubMed] [Google Scholar]
- Cox E. C., Yanofsky C. Mutator gene studies in Escherichia coli. J Bacteriol. 1969 Oct;100(1):390–397. doi: 10.1128/jb.100.1.390-397.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Charamella L. J., Berg C. M., Harris P. E. Kinetic and genetic analyses of D-cycloserine inhibition and resistance in Escherichia coli. J Bacteriol. 1965 Nov;90(5):1238–1250. doi: 10.1128/jb.90.5.1238-1250.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMEREC M., HANSON J. Mutagenic action of manganese chloride. Cold Spring Harb Symp Quant Biol. 1951;16:215–228. doi: 10.1101/sqb.1951.016.01.017. [DOI] [PubMed] [Google Scholar]
- Demerec M. Frequency of Spontaneous Mutations in Certain Stocks of Drosophila Melanogaster. Genetics. 1937 Sep;22(5):469–478. doi: 10.1093/genetics/22.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson P. T. Recombination deficient mutants of Escherichia coli K12 that map between thy A and argA. Genetics. 1968 Sep;60(1):19–30. doi: 10.1093/genetics/60.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDSTEIN A., SMOOT J. S. A strain of Escherichia coli with an unusually high rate of auxotrophic mutation. J Bacteriol. 1955 Nov;70(5):588–595. doi: 10.1128/jb.70.5.588-595.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBERG J., MANDELL J. D., WOODY P. L. Resistance and cross-resistance of Escherichia coli mutants to antitumour agent mitomycin C. J Gen Microbiol. 1961 Nov;26:509–520. doi: 10.1099/00221287-26-3-509. [DOI] [PubMed] [Google Scholar]
- Gross J. D., Karamata D., Hempstead P. G. Temperature-sensitive mutants of B. subtilis defective in DNA synthesis. Cold Spring Harb Symp Quant Biol. 1968;33:307–312. doi: 10.1101/sqb.1968.033.01.034. [DOI] [PubMed] [Google Scholar]
- HANAWALT P. C., HAYNES R. H. REPAIR REPLICATION OF DNA IN BACTERIA: IRRELEVANCE OF CHEMICAL NATURE OF BASE DEFECT. Biochem Biophys Res Commun. 1965 May 3;19:462–467. doi: 10.1016/0006-291x(65)90147-6. [DOI] [PubMed] [Google Scholar]
- HILL R. F. Independent inactivation of bacteriophage T1 by x-rays and ultraviolet light. Radiat Res. 1958 Jan;8(1):46–50. [PubMed] [Google Scholar]
- HILL R. F., ROSSI H. H. Absence of photoreactivation in T1 bacteriophage irradiated with ultraviolet in the dry state. Science. 1952 Oct 17;116(3016):424–425. doi: 10.1126/science.116.3016.424. [DOI] [PubMed] [Google Scholar]
- Haefner K. Spontaneous lethal sectoring, a further feature of Escherichia coli strains deficient in the function of rec and uvr genes. J Bacteriol. 1968 Sep;96(3):652–659. doi: 10.1128/jb.96.3.652-659.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helling R. B. Selection of a mutant of Escherichia coli which has high mutation rates. J Bacteriol. 1968 Oct;96(4):975–980. doi: 10.1128/jb.96.4.975-980.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. F. Do dark repair mechanisms for UV-induced primary damage affect spontaneous mutation? Mutat Res. 1968 Nov-Dec;6(3):472–475. doi: 10.1016/0027-5107(68)90065-1. [DOI] [PubMed] [Google Scholar]
- Hill R. F. Location of genes controlling excision repair of UV damage and mutator activity in Escherichia coli WP2. Mutat Res. 1970 Mar;9(3):341–344. doi: 10.1016/0027-5107(70)90135-1. [DOI] [PubMed] [Google Scholar]
- Hill R. F. Ultraviolet-induced lethality and reversion to prototrophy in Escherichia coli strains with normal and reduced dark repair ability. Photochem Photobiol. 1965 Jun;4(3):563–568. doi: 10.1111/j.1751-1097.1965.tb09774.x. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R. D. Metabolism and growth of gene substance: 1968. Cold Spring Harb Symp Quant Biol. 1968;33:857–870. doi: 10.1101/sqb.1968.033.01.097. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P. DNA repair and genetic recombination: studies on mutants of Escherichia coli defective in these processes. Radiat Res. 1966;(Suppl):156+–156+. [PubMed] [Google Scholar]
- IYER V. N., SZYBALSKI W. A MOLECULAR MECHANISM OF MITOMYCIN ACTION: LINKING OF COMPLEMENTARY DNA STRANDS. Proc Natl Acad Sci U S A. 1963 Aug;50:355–362. doi: 10.1073/pnas.50.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IYER V. N., SZYBALSKI W. MITOMYCINS AND PORFIROMYCIN: CHEMICAL MECHANISM OF ACTIVATION AND CROSS-LINKING OF DNA. Science. 1964 Jul 3;145(3627):55–58. doi: 10.1126/science.145.3627.55. [DOI] [PubMed] [Google Scholar]
- JYSSUM K. Observations on two types of genetic instability in Escherichia coli. Acta Pathol Microbiol Scand. 1960;48:113–120. doi: 10.1111/j.1699-0463.1960.tb04747.x. [DOI] [PubMed] [Google Scholar]
- Johnson H. G., Bach M. K. The antimutagenic action of polyamines: suppression of the mutagenic action of an E. coli mutator gene and of 2-aminopurine. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1453–1456. doi: 10.1073/pnas.55.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyssum K. Mutator factor in Neisseria meningitidis associated with increased sensitivity to ultraviolet light and defective transformation. J Bacteriol. 1968 Jul;96(1):165–172. doi: 10.1128/jb.96.1.165-172.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRCHNER C. E. The effects of the mutator gene on molecular changes and mutation of Salmonella typhimurium. J Mol Biol. 1960 Dec;2:331–338. doi: 10.1016/s0022-2836(60)80044-7. [DOI] [PubMed] [Google Scholar]
- Kirchner C. E., Rudden M. J. Location of a mutator gene in Salmonella typhimurium by cotransduction. J Bacteriol. 1966 Nov;92(5):1453–1456. doi: 10.1128/jb.92.5.1453-1456.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S., Kato T. Action spectra for photoreactivation of killing and mutation to prototrophy in U.V.-sensitive strains of Escherichia Coli possessing and lacking photoreactivating enzyme. Photochem Photobiol. 1966 Nov-Dec;5(11):827–837. doi: 10.1111/j.1751-1097.1966.tb05929.x. [DOI] [PubMed] [Google Scholar]
- LATHAM A. B., WEINBERG R. Apparent mutagenic effect of thymine deficiency for a thymine-requiring strain of Escherichia coli. J Bacteriol. 1956 Oct;72(4):570–572. doi: 10.1128/jb.72.4.570-572.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake T. Mutator Factor in Salmonella Typhimurium. Genetics. 1960 Jan;45(1):11–14. doi: 10.1093/genetics/45.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohn G., Kaplan R. W. Beeinflussung der spontanen und chemisch induzierten Mutabilität bei E.coli durch Anderung des genetischen Hintergrundes. Mol Gen Genet. 1967;99(2):191–202. doi: 10.1007/BF00426163. [DOI] [PubMed] [Google Scholar]
- Mohn G. Korrelation zwischen verminderter Reparaturfähigkeit für UV-Läsionen und hoher Spontanmutabilität eines Mutatorstammes von E.coli K-12. Mol Gen Genet. 1968;101(1):43–50. doi: 10.1007/BF00434810. [DOI] [PubMed] [Google Scholar]
- Moseley B. E. The isolation and some properties of radiation-sensitive mutants of Micrococcus radiodurans. J Gen Microbiol. 1967 Nov;49(2):293–300. doi: 10.1099/00221287-49-2-293. [DOI] [PubMed] [Google Scholar]
- Orgel A., Orgel L. E. Induction of mutations in bacteriophage T4 with divalent manganese. J Mol Biol. 1965 Dec;14(2):453–457. doi: 10.1016/s0022-2836(65)80195-4. [DOI] [PubMed] [Google Scholar]
- Otsuji N. Properties of mitomycin C-sensitive mutants of Escherichia coli K-12. J Bacteriol. 1968 Feb;95(2):540–545. doi: 10.1128/jb.95.2.540-545.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel E. C., Bryson V. Mutator gene of Escherichia coli B. J Bacteriol. 1967 Jul;94(1):38–47. doi: 10.1128/jb.94.1.38-47.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaar P. D. A BINARY MUTABILITY SYSTEM IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1956 May;42(5):245–249. doi: 10.1073/pnas.42.5.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speyer J. F. Mutagenic DNA polymerase. Biochem Biophys Res Commun. 1965 Oct 8;21(1):6–8. doi: 10.1016/0006-291x(65)90417-1. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treffers H. P., Spinelli V., Belser N. O. A Factor (or Mutator Gene) Influencing Mutation Rates in Escherichia Coli. Proc Natl Acad Sci U S A. 1954 Nov;40(11):1064–1071. doi: 10.1073/pnas.40.11.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J., Low B. Genetic location of certain mutations conferring recombination deficiency in Escherichia coli. J Bacteriol. 1969 Jan;97(1):244–249. doi: 10.1128/jb.97.1.244-249.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Radiation-induced mutations and their repair. Science. 1966 Jun 3;152(3727):1345–1353. doi: 10.1126/science.152.3727.1345. [DOI] [PubMed] [Google Scholar]


