Abstract
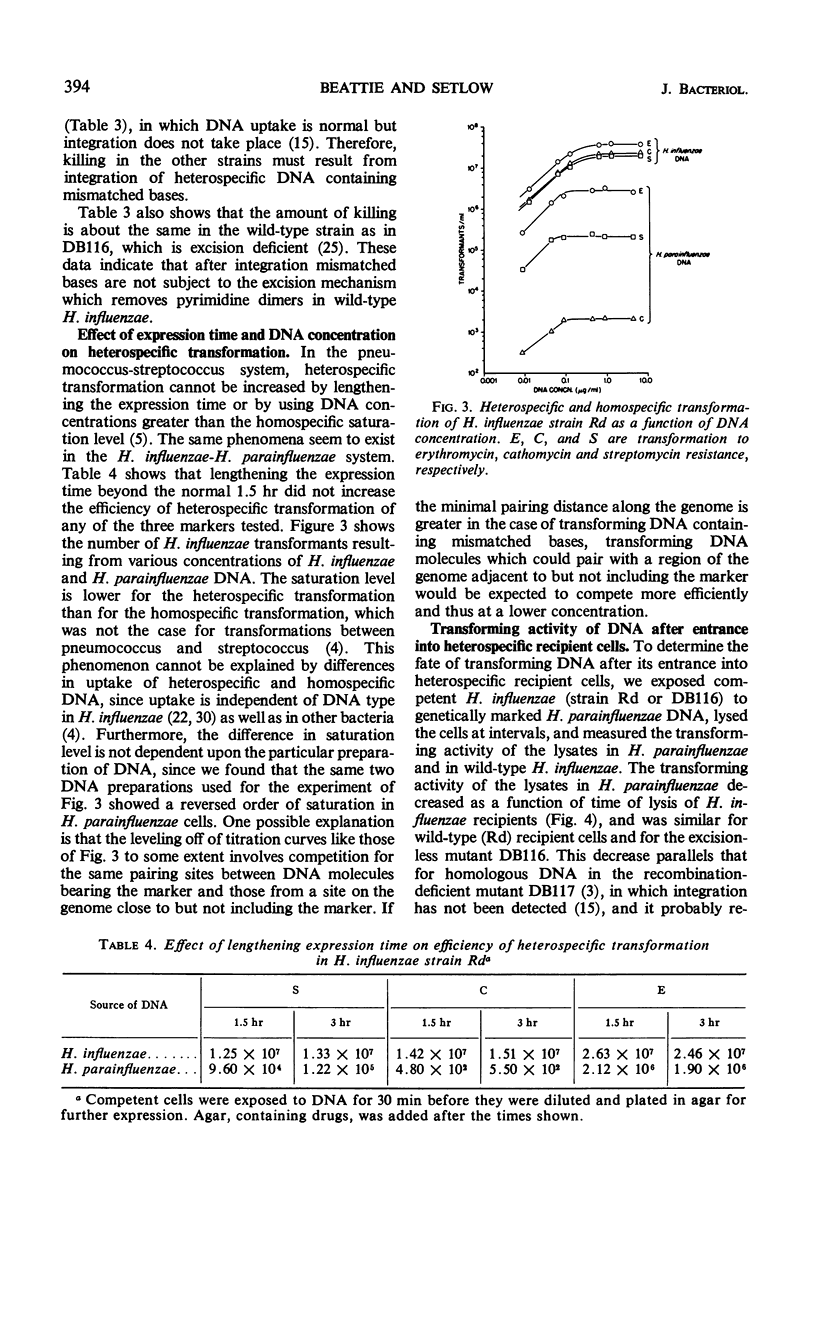
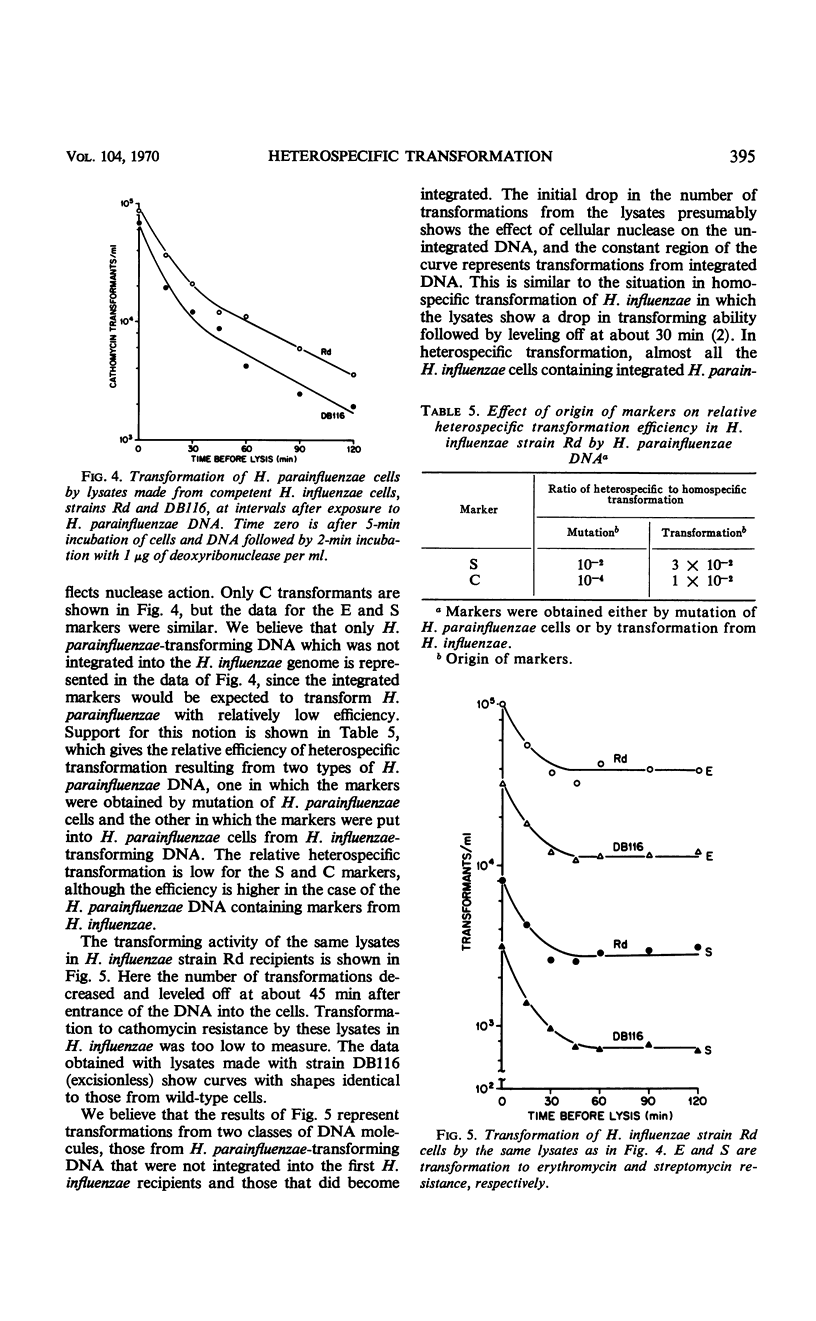
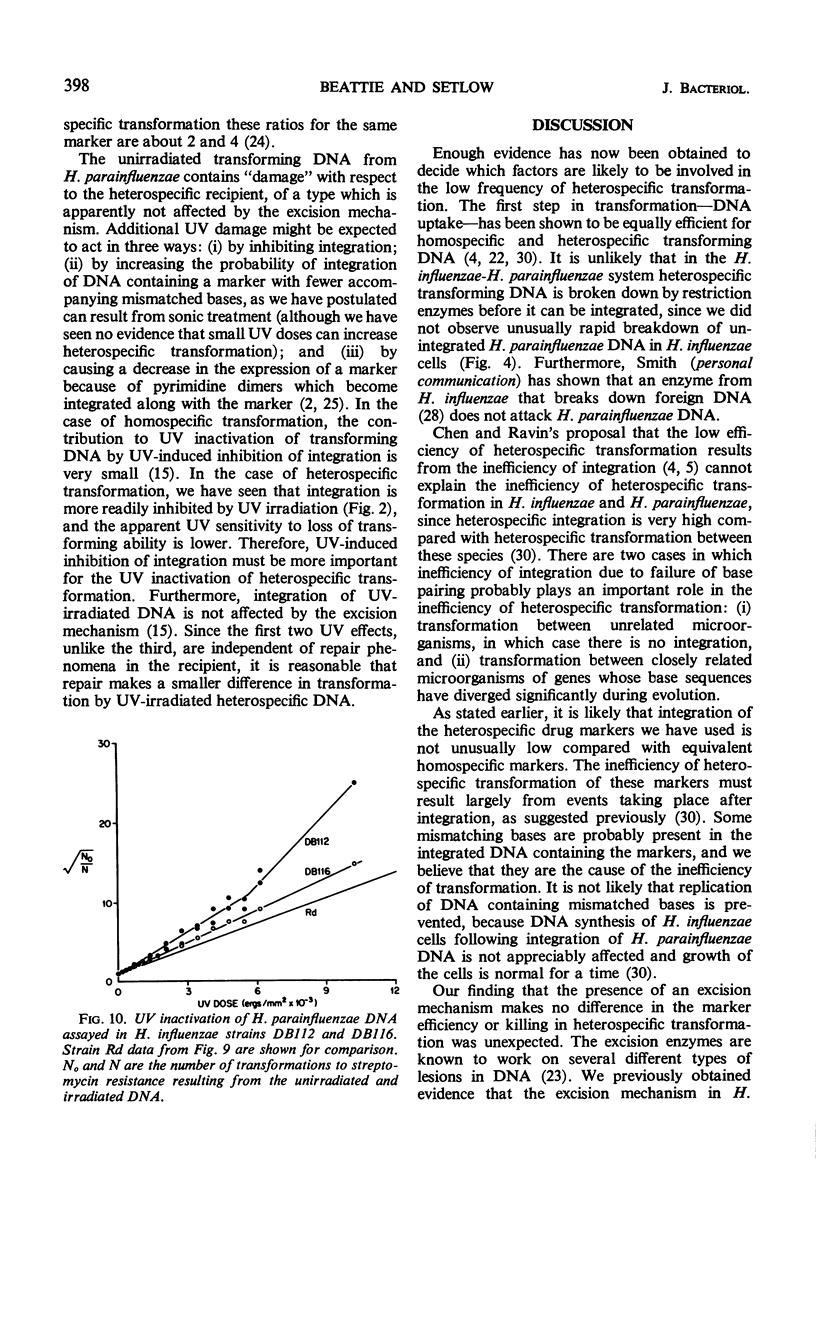
Heterospecific transformation between Haemophilus influenzae and H. parainfluenzae is from one to more than six orders of magnitude lower than homospecific transformation, depending on the marker assayed. However, the physical integration of deoxyribonucleic acid (DNA) in heterospecific compared with homospecific transformation is only slightly decreased. Measurement of integration of ultraviolet-irradiated heterospecific transforming DNA suggests that compared with homospecific DNA a longer piece of heterospecific transforming DNA must undergo pairing for integration to occur. Heterospecific transforming DNA behaves towards ultraviolet inactivation of biological activity as though it had undergone some previous inactivation. The efficiency of heterospecific transformation can be improved by light sonic treatment of the DNA or by the use of DNA containing markers which originated from the heterospecific recipient. The presence of an excision mechanism in the recipient cell does not affect killing or marker efficiency in heterospecific transformation. The data indicate that the low frequency of transformation between H. influenzae and H. parainfluenzae results mostly from lethality. It is proposed that integration of heterospecific transforming DNA results in alterations in the base sequence of the recipient genome which cannot be repaired. Transcription and translation of the altered DNA could result in synthesis of nonfunctional essential proteins.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beattie K. L., Setlow J. K. Killing of Haemophilus influenzae cells by integrated ultraviolet-induced lesions from transforming deoxyribonucleic acid. J Bacteriol. 1969 Dec;100(3):1284–1288. doi: 10.1128/jb.100.3.1284-1288.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie K. L., Setlow J. K. Repair of ultraviolet-irradiated transforming deoxyribonucleic acid in Haemophilus influenzae. J Bacteriol. 1970 Mar;101(3):808–812. doi: 10.1128/jb.101.3.808-812.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boling M. E., Setlow J. K. Dependence of Vegetative Recombination Among Haemophilus influenzae Bacteriophage on the Host Cell. J Virol. 1969 Sep;4(3):240–243. doi: 10.1128/jvi.4.3.240-243.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COX E. C., WHITE J. R., FLAKS J. G. STREPTOMYCIN ACTION AND THE RIBOSOME. Proc Natl Acad Sci U S A. 1964 Apr;51:703–709. doi: 10.1073/pnas.51.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland J. C., Marmur J. Identification of conserved genetic functions in Bacillus by use of temperature-sensitive mutants. Bacteriol Rev. 1968 Dec;32(4 Pt 1):302–312. [PMC free article] [PubMed] [Google Scholar]
- Cundliffe E., McQuillen K. Bacterial protein synthesis: the effects of antibiotics. J Mol Biol. 1967 Nov 28;30(1):137–146. doi: 10.1016/0022-2836(67)90249-5. [DOI] [PubMed] [Google Scholar]
- Davies J., Gorini L., Davis B. D. Misreading of RNA codewords induced by aminoglycoside antibiotics. Mol Pharmacol. 1965 Jul;1(1):93–106. [PubMed] [Google Scholar]
- Dubnau D., Smith I., Morell P., Marmur J. Gene conservation in Bacillus species. I. Conserved genetic and nucleic acid base sequence homologies. Proc Natl Acad Sci U S A. 1965 Aug;54(2):491–498. doi: 10.1073/pnas.54.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODGAL S. H., HERRIOTT R. M. Studies on transformations of Hemophilus influenzae. I. Competence. J Gen Physiol. 1961 Jul;44:1201–1227. doi: 10.1085/jgp.44.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modak S. P., Setlow J. K. Synthesis of deoxyribonucleic acid after ultraviolet irradiation of sensitive and resistant Haemophilus influenzae. J Bacteriol. 1969 Jun;98(3):1195–1198. doi: 10.1128/jb.98.3.1195-1198.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammed A., Setlow J. K. Ultraviolet-induced decrease in integration of Haemophilus influenzae transforming deoxyribonucleic acid in sensitive and resistant cells. J Bacteriol. 1970 Feb;101(2):444–448. doi: 10.1128/jb.101.2.444-448.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICKEL L., GOODGAL S. H. EFFECT OF INTERSPECIFIC TRANSFORMATION ON LINKAGE RELATIONSHIPS OF MARKERS IN HAEMOPHILUS INFLUENZAE AND HAEMOPHILUS PARAINFLUENZAE. J Bacteriol. 1964 Dec;88:1538–1544. doi: 10.1128/jb.88.6.1538-1544.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notani N. K., Goodgal S. H. Decrease in integration of transforming DNA of Hemophilus influenzae following ultraviolet irradiation. J Mol Biol. 1965 Sep;13(2):611–613. doi: 10.1016/s0022-2836(65)80126-7. [DOI] [PubMed] [Google Scholar]
- Notani N., Goodgal S. H. On the nature of recombinants formed during transformation in Hemophilus influenzae. J Gen Physiol. 1966 Jul;49(6):197–209. doi: 10.1085/jgp.49.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn R. O., Setlow J. K., Hosszu J. L. Ultraviolet inactivation and photoproducts of transforming DNA irradiated at low temperatures. Biophys J. 1969 Apr;9(4):510–517. doi: 10.1016/S0006-3495(69)86401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravin A. W., Chen K. C. Heterospecific transformation of pneumococcus and streptococcus. 3. Reduction of linkage. Genetics. 1967 Dec;57(4):851–864. doi: 10.1093/genetics/57.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAEFFER P. La pénétration de l'acide nucleique dans les bactéries réceptrices au cours des transformations interspécifiques. C R Hebd Seances Acad Sci. 1957 Jul 17;245(3):375–377. [PubMed] [Google Scholar]
- SETLOW R. B., SETLOW J. K. Evidence that ultraviolet-induced thymine dimers in DNA cause biological damage. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1250–1257. doi: 10.1073/pnas.48.7.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Brown D. C., Boling M. E., Mattingly A., Gordon M. P. Repair of deoxyribonucleic acid in Haemophilus influenzae. I. X-ray sensitivity of ultraviolet-sensitive mutants and their behavior as hosts to ultraviolet-irradiated bacteriophage and transforming deoxyribonucleic acid. J Bacteriol. 1968 Feb;95(2):546–558. doi: 10.1128/jb.95.2.546-558.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Randolph M. L., Boling M. E., Mattingly A., Price G., Gordon M. P. Repair of DNA in Haemophilus influenzae. II. Excision, repair of single-strand breaks, defects in transformation, and host cell modification in UV-sensitive mutants. Cold Spring Harb Symp Quant Biol. 1968;33:209–218. doi: 10.1101/sqb.1968.033.01.024. [DOI] [PubMed] [Google Scholar]
- Setlow R. B., Carrier W. L. Pyrimidine dimers in ultraviolet-irradiated DNA's. J Mol Biol. 1966 May;17(1):237–254. doi: 10.1016/s0022-2836(66)80105-5. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Wilcox K. W. A restriction enzyme from Hemophilus influenzae. I. Purification and general properties. J Mol Biol. 1970 Jul 28;51(2):379–391. doi: 10.1016/0022-2836(70)90149-x. [DOI] [PubMed] [Google Scholar]
- Steinhart W. L., Herriott R. M. Fate of recipient deoxyribonucleic acid during transformation in Haemophilus influenzae. J Bacteriol. 1968 Nov;96(5):1718–1724. doi: 10.1128/jb.96.5.1718-1724.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhart W. L., Herriott R. M. Genetic integration in the heterospecific transformation of Haemophilus influenzae cells by Haemophilus parainfluenzae deoxyribonucleic acid. J Bacteriol. 1968 Nov;96(5):1725–1731. doi: 10.1128/jb.96.5.1725-1731.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


