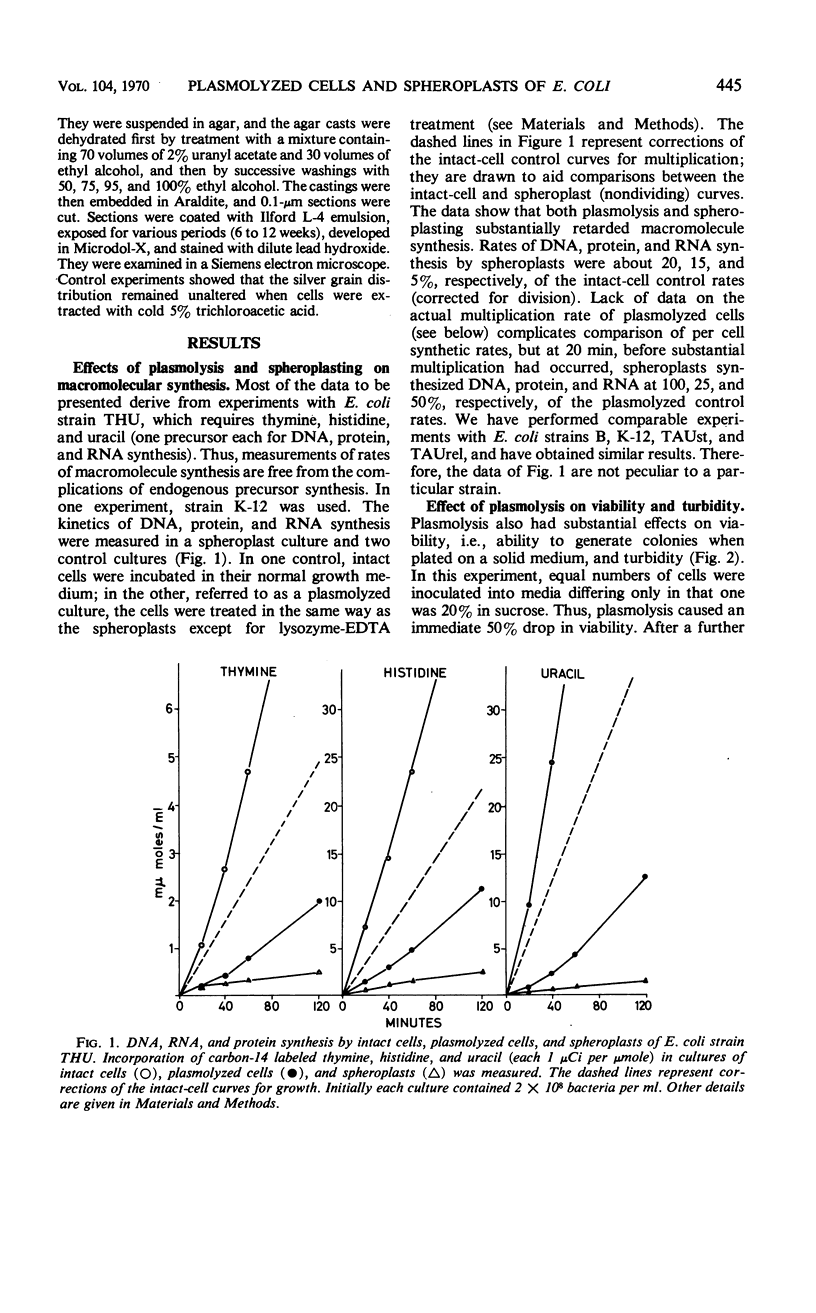
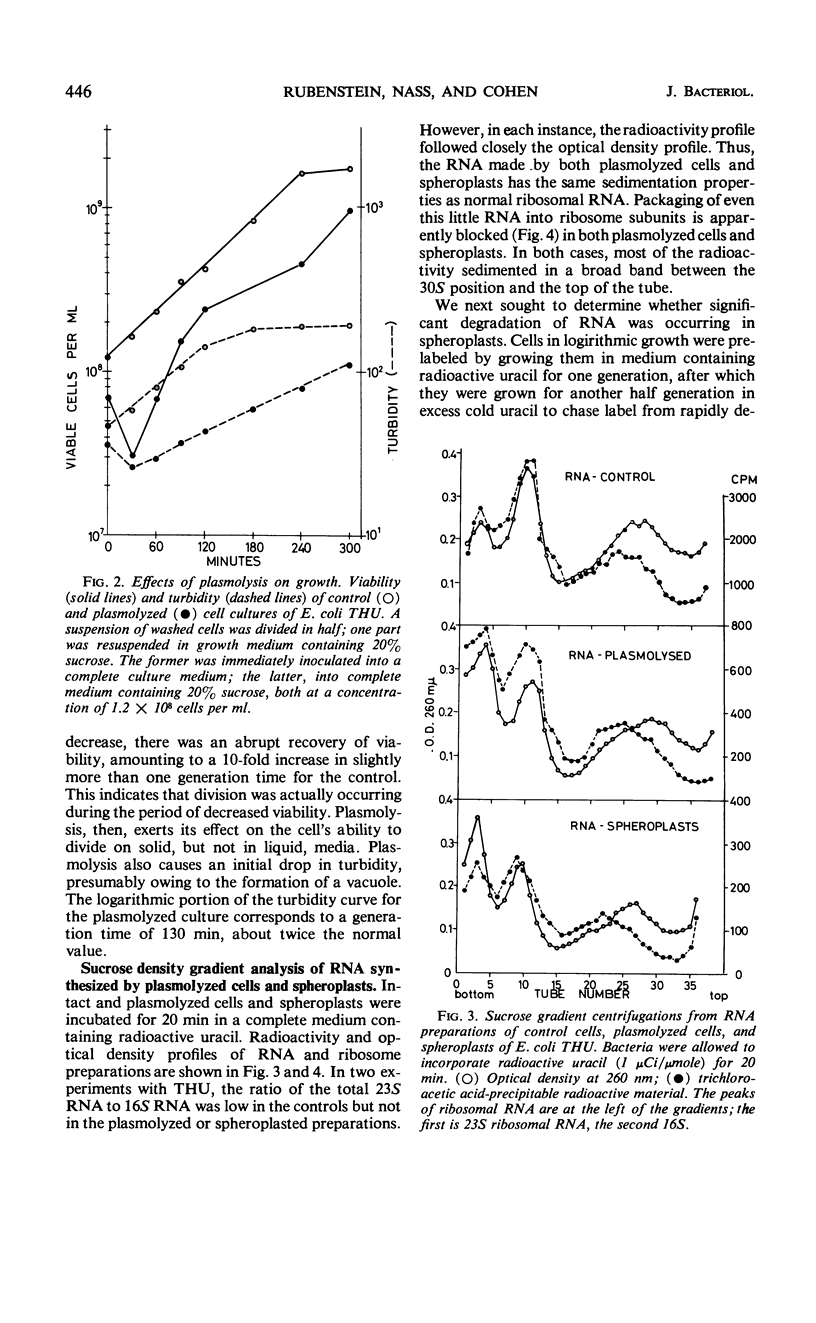
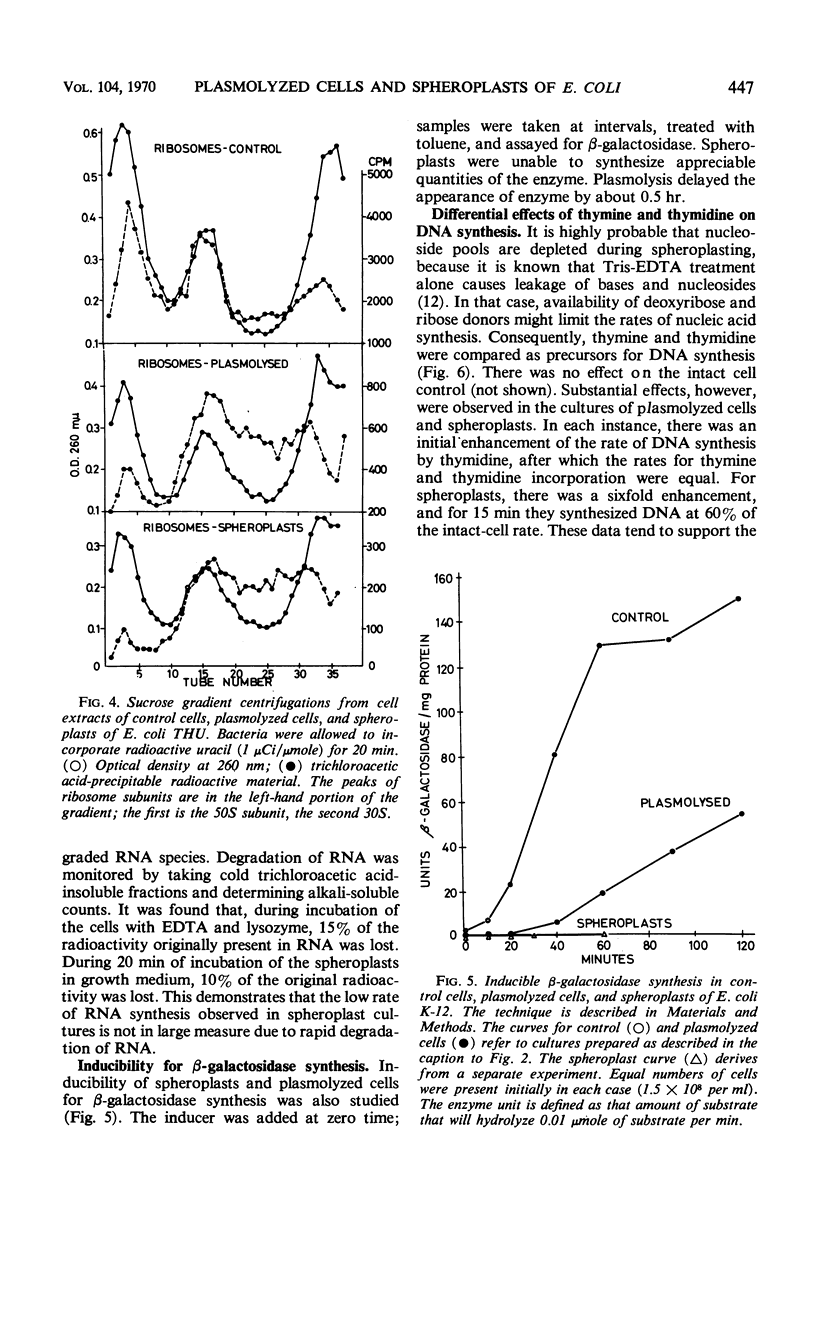
Abstract
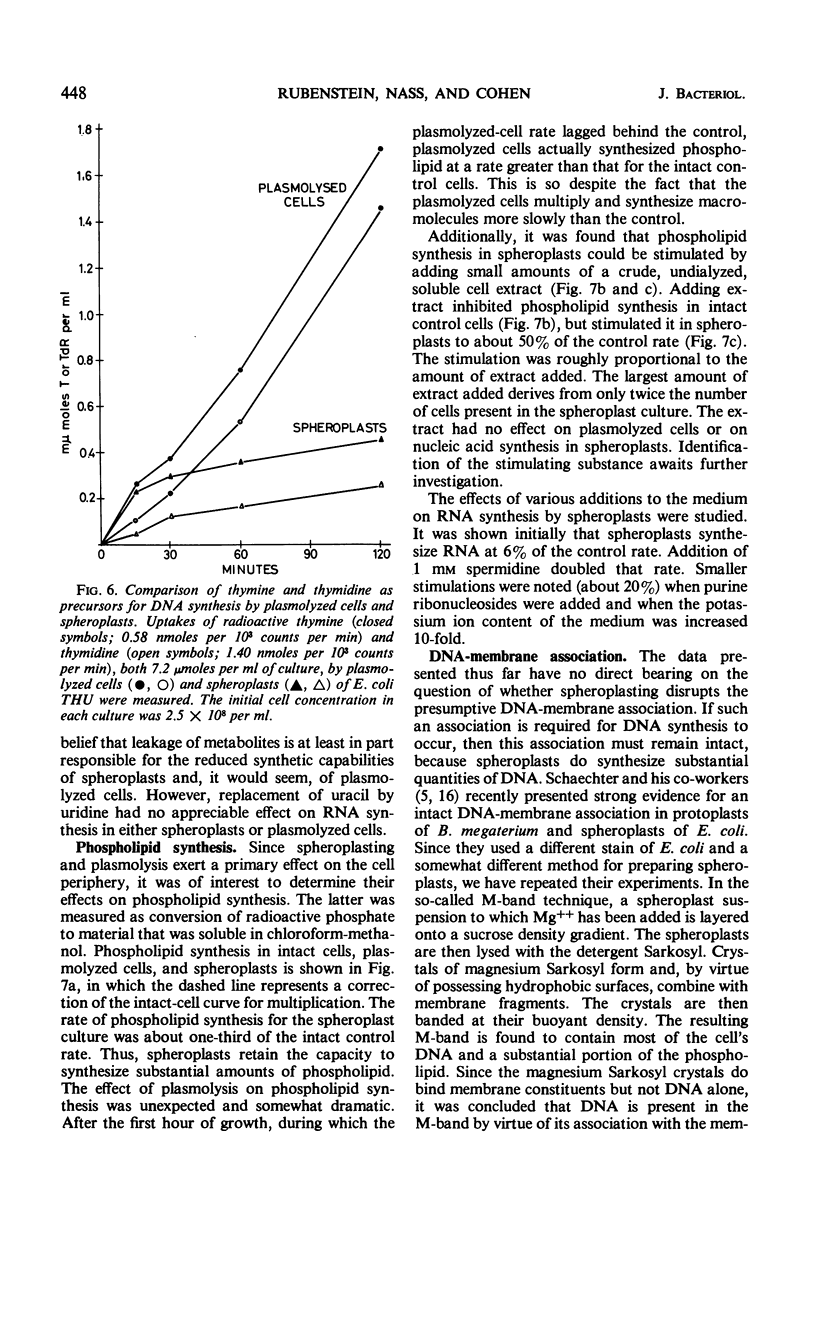
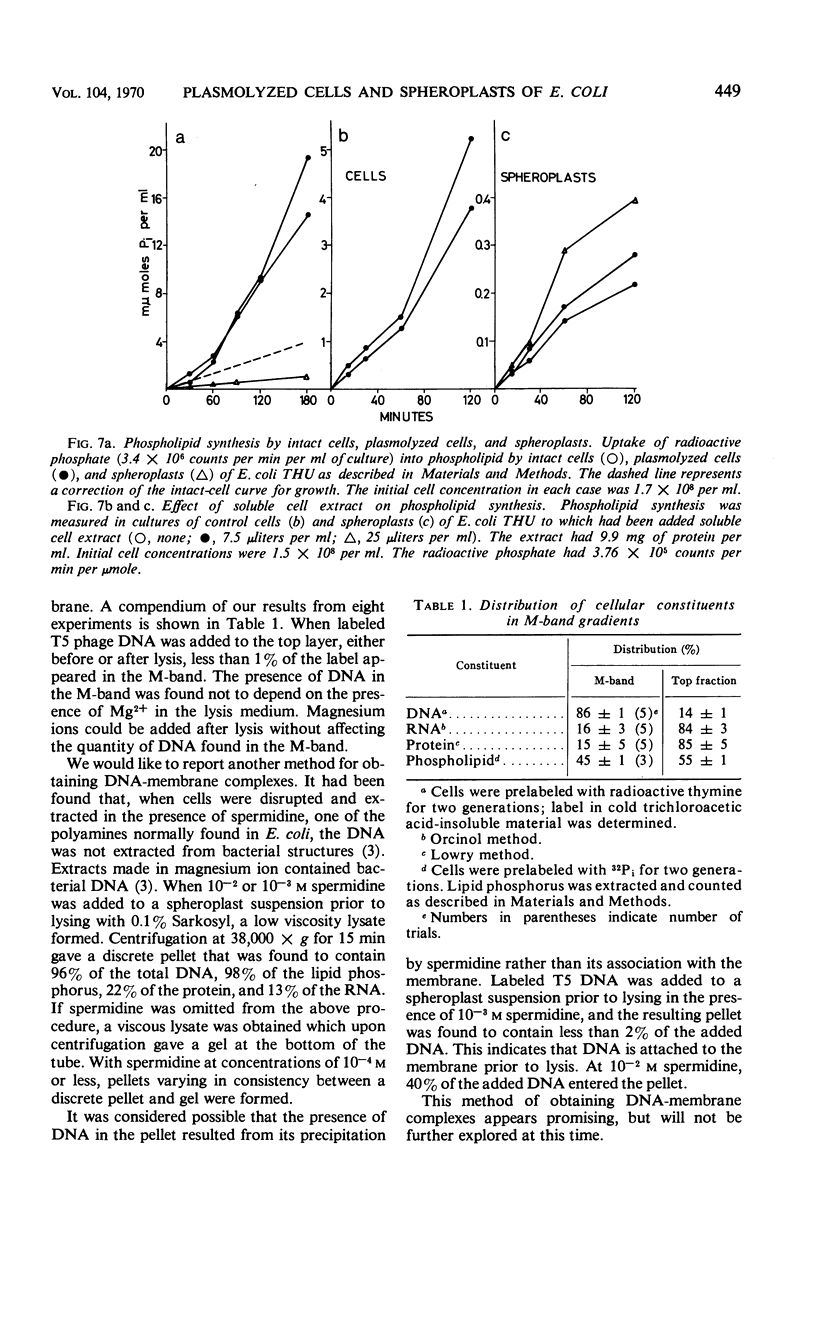
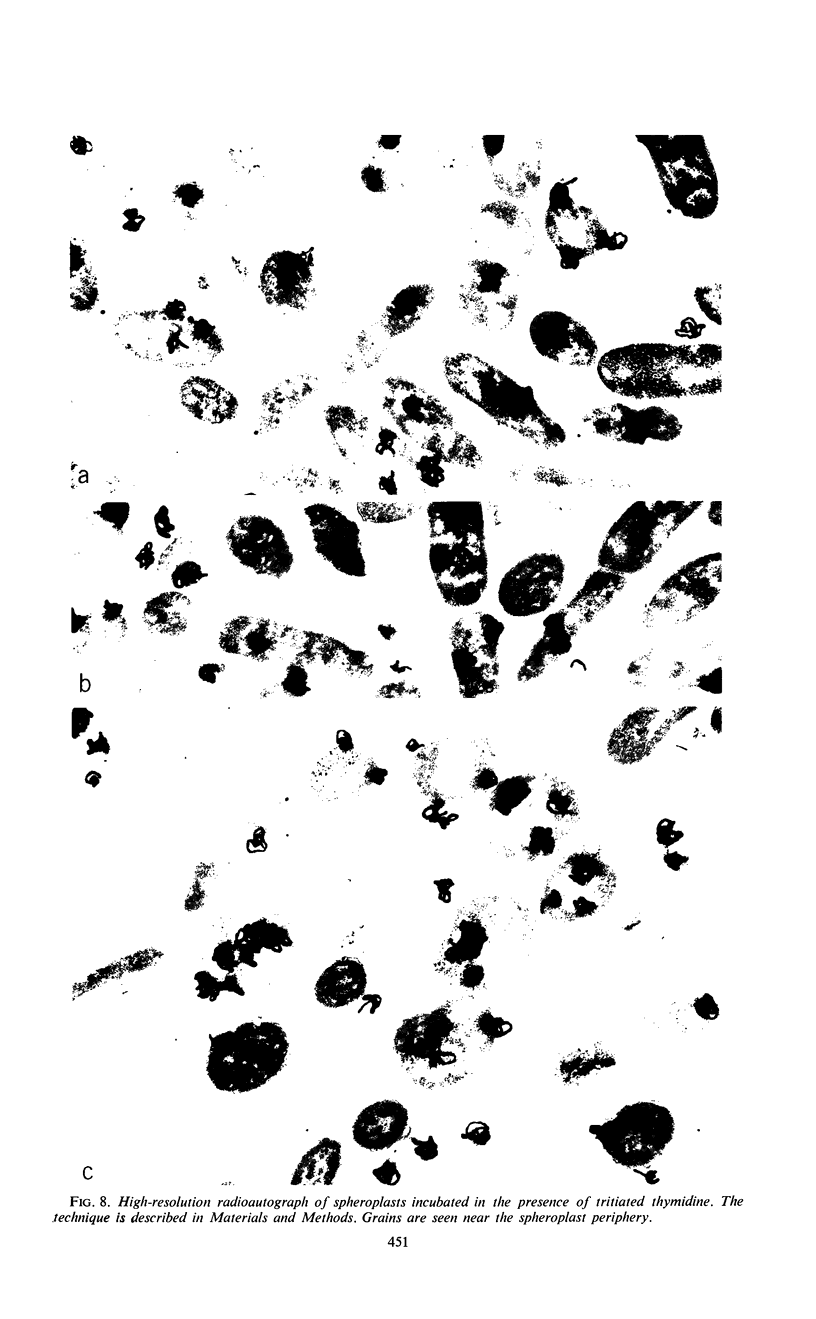
Effects of plasmolysis and spheroplast formation on deoxyribonucleic acid (DNA), ribonucleic acid (RNA), protein, and phospholipid synthesis by Escherichia coli strain THU were studied. RNA and protein synthesis were severely diminished. DNA and phospholipid synthesis were inhibited, but less so; they could be partly restored. DNA synthesis could be restored by replacing thymine in the medium with thymidine, and phospholipid synthesis, by adding back small quantities of soluble cell extract. Plasmolysis effected marked reductions in rates of growth and macro-molecule synthesis, and temporarily reduced culture viability. Plasmolysis also caused an anomalous stimulation of phospholipid synthesis. Spheroplasts and plasmolyzed cells synthesized small amounts of ribosomal RNA that sedimented normally. However, this ribosomal RNA was very inefficiently packaged to ribosome subunits. Spheroplasts were unable to carry out induced synthesis of β-galactosidase, and plasmolyzed cells were delayed in this function. Radioautographs examined in an electron microscope showed that DNA synthesis in plasmolyzed cells and spheroplasts was performed by a substantial fraction of the culture populations. That DNA and membrane were associated in the spheroplasts used in this study was suggested by formation of M-bands containing membrane and most of the cell's DNA. The results are discussed in terms of alterations of membrane structure and conformation attending plasmolysis and spheroplasting.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsell D. C., Cota-Robles E. H. Production and ultrastructure of lysozyme and ethylenediaminetetraacetate-lysozyme spheroplasts of Escherichia coli. J Bacteriol. 1967 Jan;93(1):427–437. doi: 10.1128/jb.93.1.427-437.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN S. S., LICHTENSTEIN J. Polyamines and ribosome structure. J Biol Chem. 1960 Jul;235:2112–2116. [PubMed] [Google Scholar]
- Cundliffe E. Preparation and some properties of active protoplasts of Bacillus megaterium. J Gen Microbiol. 1968 Oct;53(3):425–430. doi: 10.1099/00221287-53-3-425. [DOI] [PubMed] [Google Scholar]
- Earhart C. F., Tremblay G. Y., Daniels M. J., Schaechter M. DNA replication studied by a new method for the isolation of cell membrane-DNA complexes. Cold Spring Harb Symp Quant Biol. 1968;33:707–710. doi: 10.1101/sqb.1968.033.01.079. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld E. R., Koch A. L. RNA synthesis in penicillin spheroplasts of Escherichia coli. Biochim Biophys Acta. 1968 Nov 20;169(1):44–57. doi: 10.1016/0005-2787(68)90007-5. [DOI] [PubMed] [Google Scholar]
- Gros F., Gallant J., Weisberg R., Cashel M. Decryptification of RNA polymerase in whole cells of Escherichia coli. J Mol Biol. 1967 May 14;25(3):555–557. doi: 10.1016/0022-2836(67)90206-9. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARK C., LARK K. G. Nucleic acid synthesis in penicillin-treated Alcaligenes faecalis. J Bacteriol. 1958 Dec;76(6):666–667. doi: 10.1128/jb.76.6.666-667.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruo B., Seto H., Nagata Y. Inducible synthesis of beta-galactosidase in disrupted spheroplast of Escherichia coli. J Bacteriol. 1969 Oct;100(1):209–214. doi: 10.1128/jb.100.1.209-214.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Ashman D. F., Price T. D. Effect of ethylenediaminetetraacetic acid-Tris(hydroxymethyl)aminomethane on release of the acid-soluble nucleotide pool and on breakdown of ribosomal ribonucleic acid in Escherichia coli. J Bacteriol. 1967 Apr;93(4):1360–1368. doi: 10.1128/jb.93.4.1360-1368.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STERN J. L., SEKIGUCHI M., BARNER H. D., COHEN S. S. THE SYNTHESIS OF MESSENGER RNA WITHOUT PROTEIN SYNTHESIS. I. STUDIES WITH THYMINELESS STRAINS OF ESCHERICHIA COLI. J Mol Biol. 1964 May;8:629–637. doi: 10.1016/s0022-2836(64)80113-3. [DOI] [PubMed] [Google Scholar]
- Tremblay G. Y., Daniels M. J., Schaechter M. Isolation of a cell membrane-DNA-nascent RNA complex from bacteria. J Mol Biol. 1969 Feb 28;40(1):65–76. doi: 10.1016/0022-2836(69)90296-4. [DOI] [PubMed] [Google Scholar]