Abstract
Chlorophyllase (Chlase) is the first enzyme involved in chlorophyll (Chl) degradation and catalyzes the hydrolysis of ester bond to yield chlorophyllide and phytol. In the present study, we isolated the Chlase cDNA. We synthesized degenerate oligo DNA probes based on the internal amino acid sequences of purified Chlase from Chenopodium album, screened the C. album cDNA library, and cloned a cDNA (CaCLH, C. album chlorophyll-chlorophyllido hydrolase). The deduced amino acid sequence (347 aa residues) had a lipase motif overlapping with an ATP/GTP-binding motif (P-loop). CaCLH possibly was localized in the extraplastidic part of the cell, because a putative signal sequence for endoplasmic reticulum is at the N terminus. The amino acid sequence shared 37% identity with a function-unknown gene whose mRNA is inducible by coronatine and methyl jasmonate (MeJA) in Arabidopsis thaliana (AtCLH1). We expressed the gene products of AtCLH1 and of CaCLH in Escherichia coli, and they similarly exhibited Chlase activity. Moreover, we isolated another full-length cDNA based on an Arabidopsis genomic fragment and expressed it in E. coli, demonstrating the presence of the second Arabidopsis CLH gene (AtCLH2). No typical feature of signal sequence was identified in AtCLH1, whereas AtCLH2 had a typical signal sequence for chloroplast. AtCLH1 mRNA was induced rapidly by a treatment of MeJA, which is known to promote senescence and Chl degradation in plants, and a high mRNA level was maintained up to 9 h. AtCLH2, however, did not respond to MeJA.
More than a billion tons of chlorophyll (Chl) are estimated to degrade every year on the earth (1). The breakdown of Chl takes place not only in the normal turnover of Chl, but also at specific stages of plant development, such as leaf senescence and fruit ripening (2, 3). These phenomena would suggest that Chl degradation is strictly controlled by environmental and developmental factors. However, the molecular mechanism has long been poorly understood especially compared with Chl biosynthesis. The main reason for this is that the genes encoding the enzymes involved in Chl degradation have not been cloned, and molecular biological studies thus have been impossible.
In recent years, many Chl catabolites have been identified, and an outline of the Chl-degradation pathway has been obtained (for the most recent review, see ref. 4). In the most recently proposed pathway, the early steps of Chl degradation take place in chloroplast (4): Chl is converted to pheophorbide by the detachment of phytol and magnesium atom, and pheophorbide is degraded further by an oxygenolytic opening of the porphyrin macrocycle and a reduction of the double bond in the δ-methine bridge. Fluorescent Chl catabolites then are exported from chloroplast and modified slightly in cytosol and then imported into vacuole (4).
Chlorophyllase (chlorophyll-chlorophyllido hydrolase, Chlase; EC 3.1.1.14), which catalyzes the hydrolysis of Chl to yield chlorophyllide and phytol, is thought to be the first enzyme in the Chl-degradation pathway (4, 5). Chlase activity was discovered about 90 years ago, and Chlase has been known as one of the oldest enzymes found in plants (6). Thus, many studies have been done on Chlase, and it has been considered to exist in higher plants, diatoms, and Chlorella. The level of Chlase activity is affected by various internal and external factors (for a recent review, see ref. 7). It was shown that ethylene treatment enhanced the de novo synthesis of Chlase protein in Citrus fruit peel (8). The enzymes have been purified from various species, and their molecular masses, as estimated by SDS/PAGE, ranged from 27 to 46 kDa. For example, Chlase from Chlorella protothecoides was shown to be a 38-kDa protein (9). Chlase purified from a diatom, Phaeodactylum tricornutum, showed two bands, a major band estimated to be 43 kDa and a minor band of 46 kDa (10). The molecular mass of Chlases from Citrus was reported to be 35 and 27 kDa, depending on the species (8, 11). It is unknown, however, whether the difference in their molecular masses is due to the difference of plant species or to the existence of different types of isozymes, or both.
Chlase has been considered to be a membrane protein in chloroplast and to exist in thylakoid membrane (12, 13). It was shown recently that Chlase activity also existed in the envelope membrane fraction of chloroplast (14, 15). In the diatom P. tricornutum, Chlase was found as a glycoprotein (16, 17), suggesting a modification (glycosylation) in endoplasmic reticulum (ER).
We have studied Chl degradation by using leaves of Chenopodium album as plant material because the extracts of the Chenopodium leaves degrade Chl more actively than other plant species do (5, 18–20). Recently, from C. album leaves we purified three types of Chlase of slightly different molecular weight on SDS/PAGE after separation by Mono Q ion-exchange chromatography (21). Two major Chlases (types 1 and 2) were purified to homogeneity, and N-terminal sequences were determined (21). In the present study, we determined the internal amino acid sequences of these Chlases and isolated a Chlase gene from C. album. Moreover, we also identified two Chlase gene homologues in Arabidopsis thaliana that respond differently to methyl jasmonate (MeJA), a plant growth regulator promoting senescence and Chl degradation in plants.
Materials and Methods
Peptide Sequencing.
Three C. album Chlases described in a previous report (21) were separated on SDS-polyacrylamide gel and blotted onto a poly(vinylidene difluoride) membrane (ProBlott; Applied Biosystems). Proteins of Chlases were digested with Achromobacter protease I by the method of Iwamatsu (22). Peptide fragments were separated by reverse-phase HPLC, and, subsequently, the amino acid sequences were determined by using a gas-phase sequencer (Model PPSQ23; Shimadzu).
Construction of a cDNA Library.
The total RNA was extracted from mature leaves of C. album by the acid guanidinium thiocyanate method (23). Poly(A)+ RNA was isolated by using oligo(dT)-Latex (TaKaRa Shuzo, Kyoto). A cDNA library was constructed from poly(A)+ RNA by using the ZAP-cDNA synthesis kit (Stratagene) as instructed by the manufacturer.
Screening of cDNA.
Degenerate oligo DNA probes were synthesized based on internal peptide sequences of three C. album Chlases. Several probes were used for screening of the cDNA library, and positive clones were obtained by using a probe (5′-ACRAARTGISWISWRTTYTCYT-3′, International Union of Pure and Applied Chemistry code). This probe corresponds to the amino acid sequence, KENSSHFV. Hybridizations were carried out at 37°C in 6× standard saline citrate (SSC)/5× Denhardt's solution containing 100 μg of denatured salmon sperm DNA per ml. Membranes were washed with 6× SSC/0.1% SDS at 40°C and autoradiographed. pBluescript phagemids with insert were obtained from positive λ-phage clones by the in vivo excision method according to the manufacturer's instructions (Stratagene). For the isolation of Chlase cDNAs of A. thaliana, a cDNA library constructed from whole rosette leaves grown in continuous light for 4–5 weeks was screened.
DNA Sequencing.
DNAs were sequenced by using a Thermo Sequenase cycle sequencing kit (Amersham Pharmacia) and a DNA sequencer (model 4000L; Li-Cor, Lincoln, NE).
Southern Blot Analysis.
Genomic DNA was prepared from A. thaliana by the method of Li and Chory (24). Five micrograms of genomic DNA was digested with restriction enzymes. They were separated on agarose gel and transferred onto nylon membrane (NYTRAN; Schleicher & Schuell). We performed hybridization analyses by using PCR-amplified and 32P-labeled probes specific for both AtCLHs containing 3′ UTR regions (891–1172 for AtCLH1, AF021244; 885-1112 for AtCLH2, AF134302).
Northern Blot Analysis.
Twenty micrograms of total RNA prepared from A. thaliana (23) was electrophoresed on 1.2% agarose/formaldehyde gel and blotted onto a nylon membrane by capillary transfer and hybridized. Specific probes for both AtCLHs described above were used.
Protein Expression in Escherichia coli.
Chlase genes were overexpressed in E. coli by using the pET expression system (Novagen). Chlase expression plasmids were constructed by PCR. The forward primers were designed to contain an extra NdeI restriction site at the 5′ end, and the reverse primers were designed to contain a stop codon and an extra XhoI restriction site (5′-CATATGTCTGAAAAAATTACTGATGTTTTCCATAAG-3′, 5′-CTCGAGTTAAAGTTGAGCATAAGTTGTTGCAAAC-3′ for CaCLH; 5′-CATATGGCGGCGATAGAGGACAG-3′, 5′-CTCGAGCTAGACGAAGATACCAGAAGCTTCTTC-3′ for AtCLH1; 5′-CATATGTCCTCTTCTTCATCAAGAAAC-3′, 5′-CTCGAGTTACATGATAACCTCAAACTCTTG-3′ for AtCLH2). PCR products were sequenced and digested by NdeI and XhoI restriction enzymes. Digested fragments were subcloned into T7 polymerase expression vector pET-24b. E. coli strain BL21(DE3) was transformed with these plasmids and used for expression. Control E. coli was transformed with pET-24b vector without insert. Transformants were inoculated in LB medium containing 50 μg/ml kanamycin and cultured at 37°C until OD600 reached an appropriate value. Recombinant Chlases were induced by the addition of isopropyl β-d-thiogalactopyranoside to a final concentration of 1 mM. Subsequent protein expression was allowed to proceed for 16 h at 20°C before a harvesting of cells by centrifugation at 10,000 × g for 10 min at 4°C. The cells were resuspended in 50 mM Mops buffer (pH 7.8) and sonicated repeatedly, and aliquots of extracts were assayed.
Enzyme Assay.
Chlase activity was determined by measuring the increase of A667 resulting from the formation of chlorophyllide a in the phase-separated aqueous-acetone layer as described previously (21).
Plant Growth and Treatment.
Wild-type A. thaliana ecotype Columbia was grown for 3 weeks at 22°C on agar plates containing MS medium (25) under 16-h light/8-h dark conditions. The plants with roots then were transferred to MS medium and kept for 12 h to avoid the effect of transient stress caused by the transfer. Then, 10 mM MeJA in DMSO was added to Murashige-Skoog (MS) medium up to the final concentration of 10 μM MeJA. At the indicated times from the start of the treatment, rosette leaves were frozen in liquid nitrogen and stored at −80°C until use.
Results
Cloning, Sequencing, and Overexpression of the C. album Chlase cDNA.
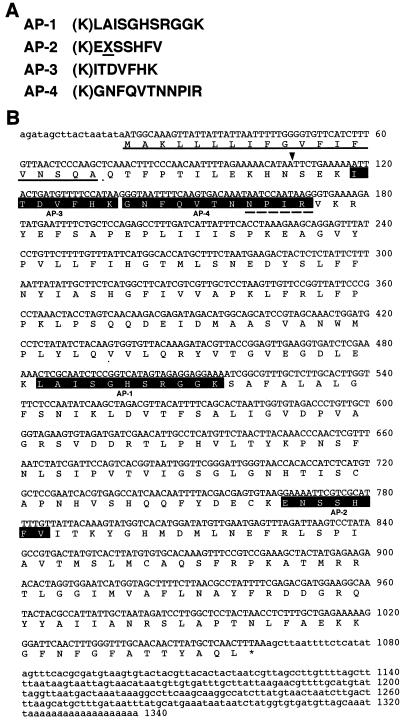
We purified the three types of Chlase (type 1, type 2, and type 0; type 0 is a more basic component in Mono Q chromatography) from mature leaves of C. album as described before (21) and determined internal peptide sequences for each Chlase. Based on the peptide sequences of these Chlases, we synthesized various degenerate oligo DNA probes and screened the cDNA library with these probes; we obtained a cDNA clone (1,340 bp in length as the longest clone) by using the probe described in Materials and Methods. The probe corresponded to a conserved and suitable peptide sequence among the internal peptides of the three Chlases (AP-2 in Fig. 1A for types 0 and 1; “X” is Asn for type 2). The amino acid sequence deduced from the nucleic acid sequence is shown in Fig. 1B, and the sequences determined by the peptide sequencing are highlighted, indicating that this clone corresponds to type 0 Chlase. We named this gene CaCLH (C. album chlorophyll-chlorophyllido hydrolase). Although we could not determine the N-terminal sequence of type 0 Chlase by amino acid sequencing (21), we assumed Ser-31 to be a putative mature N terminus of CaCLH from the results of the N-terminal sequencing for types 1 and 2 Chlases (ref. 21 and arrowhead in Fig. 1B). In spite of several screenings, no clone was obtained for types 1 and 2. A reason for this might be that the population of transcripts for types 1 and 2 is much lower than that of type 0 Chlase in spite of the large population of proteins.
Figure 1.
(A) Internal peptide sequences of the purified C. album Chlase (type 0). The amino acid expressed as “X” and underlined in AP-2 is the putative modifiable amino acid (see text). (B) Nucleotide and deduced amino acid sequences of CaCLH. The internal peptide sequences shown in A are highlighted. The putative signal sequence for ER is underlined, and the putative cleavage site for mature protein is indicated by the arrowhead. The dashed underline indicates the sequence identical to NPIR central motif, a vacuolar-sorting determinant conserved in prosporamin and proaleurain (26).
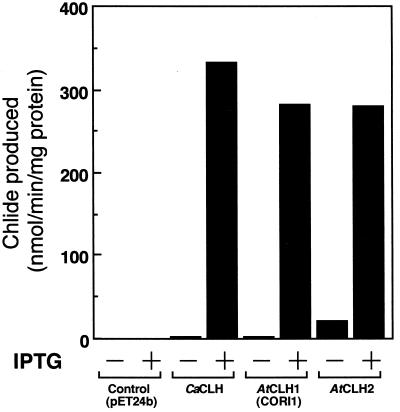
A putative mature protein region of CaCLH was subcloned to pET-24b vector, and the recombinant CaCLH was expressed in E. coli. The extract of CaCLH-expressing E. coli had a lot of Chlase activity, whereas the extract of control E. coli had none (Fig. 2), confirming that the isolated cDNA encoded C. album Chlase. SDS/PAGE of the E. coli extract showed that the induced band corresponding to the recombinant CaCLH (estimated molecular weight, 35,290) was about a 36-kDa protein. Because the native Chlase (type 0, CaCLH) purified from C. album was electrophoresed as a slightly larger protein (42 kDa; unpublished result), the native Chlase (CaCLH) possibly was modified in vivo. We will discuss this possibility later.
Figure 2.
Chlase activity of the recombinant CLH proteins. CLHs were expressed in E. coli without tag fusion. Control E. coli was transformed with pET-24b vector without insert. Aliquots of E. coli extracts were assayed for Chlase activity. IPTG, isopropyl β-d-thiogalactoside.
Identification of Two Chlase Homologues from A. thaliana.
The deduced amino acid sequence of CaCLH shared 37% homology with that of a previously reported Arabidopsis gene, CORI1 (AF021244), the mRNA of which is inducible by coronatine (27). Although the function of the gene product had not been determined, its mRNA expression also was induced by MeJA, which is known as a senescence-promoting plant growth regulator (27). Several regions in CaCLH and CORI1 were highly conserved, especially in the lipase motif (see next section) (Fig. 3). To identify this gene as a Chlase in A. thaliana, we isolated the cDNA from a cDNA library and expressed the protein in E. coli. The recombinant CORI1 product had Chlase activity at a level similar to that of CaCLH (Fig. 2). Thus, we concluded that this gene product was certainly a Chlase of A. thaliana (A. thaliana CLH1, AtCLH1). Furthermore, we also found a sequence of a genomic fragment (641 bp, 1884xa.pcr, AF005802) homologous to AtCLH1 (28) and showed the deduced amino acid sequence of this genomic fragment to have high homology with the well conserved regions between CaCLH and AtCLH1. We therefore assumed the gene product to be another Chlase isozyme in A. thaliana and isolated the corresponding full-length cDNA. The obtained cDNA was 1,135-bp long, and the deduced amino acid sequence had meaningful homology to CaCLH and AtCLH1 (Fig. 3). The product of this novel gene was expressed in E. coli, and significant Chlase activity also was observed in the extract of the transformant (Fig. 2). Thus, we named this gene product A. thaliana CLH2 (AtCLH2).
Figure 3.
Multiple alignment of the deduced amino acid sequences of CLHs. The amino acids identical among all three CLHs are highlighted, and the similar amino acids are shaded in gray. The putative N-terminal cleavage site of CaCLH is shown by the downward arrow. The lipase motif sequence is shown with a dashed underline, and the P-loop sequence is underlined. The putative active site Ser residue is indicated by a star. The intron insertion position conserved between AtCLHs is indicated by the upward arrow.
Comparison of Deduced Amino Acid Sequences Between Three CLHs.
The multiple alignment of the deduced amino acid sequences of these three CLHs is shown in Fig. 3. Homology among these CLHs was low (32–40%) in whole sequence, but several regions were highly conserved (Fig. 3). In the most conserved region, two motifs were found by using a prosite database (29). One was the lipase motif (Ser active site), [LIV]-x-[LIVFY]-[LIVMST]-G-[HYWV]-S-x-G-[GSTAC] (PS00120). As to AtCLHs, the dashed, underlined region in Fig. 3 was identical to the lipase motif (Ser active site) except for the first amino acid residue (T, F). Because Chlase hydrolyzes the ester bond between carboxylic acid and alcohol, this lipase motif region probably functions as a catalytic region in CLHs. In fact, it was reported that Chlase activity was inhibited by diisopropyl fluorophosphate, a serine enzyme inhibitor (10), and we also confirmed 65% inhibition of partially purified C. album Chlases by 1.25 mM PMSF, a serine protease inhibitor. The conserved serine residue marked by a star in Fig. 3 is probably an active site of CLHs. The other ATP/GTP-binding site motif A, [AG]-x-x-x-x-G-K-[ST] (P-loop, PS00017), was overlapped partially with the lipase motif (underlined in Fig. 3), but it is unknown whether ATP or GTP binds to CLH. Chlase has been considered as a membrane protein (4, 15), but no potential membrane spanning region was found in any of the CLHs cloned.
Although C. album is a good source of Chlase (21), the plant is not suitable for molecular biological studies because of the difficulty in controlling its growth in the laboratory. Thus, for further analysis, we used A. thaliana as a plant material. To reduce the possibility of cross-hybridization, we prepared specific probes for both AtCLHs by PCR. Genomic Southern analysis for AtCLHs showed a single band in individual cases of restriction enzymes for each CLH (Fig. 4A), indicating that these CLHs existed as a single copy on the Arabidopsis genome. Our PCR analysis revealed that only one intron was present in the coding region in the genomic sequences of AtCLH1 and AtCLH2, and the position of the intron insertion was conserved (upward arrow in Fig. 3). This suggested that these two CLHs were closely associated with each other in the early steps of their evolution, although they showed low homology in amino acid sequences. Recently, the genome project of A. thaliana revealed that AtCLH1 and AtCLH2 were in chromosomes 1 and 5, respectively.
Figure 4.
(A) Genomic Southern analysis of AtCLHs. Genomic DNA was digested by the indicated restriction enzymes and hybridized with the specific probes for AtCLHs. (B) Effect of MeJA on mRNA accumulation of AtCLHs. The number above each lane indicates the hours of MeJA treatment. Seedlings were treated with 10 μM MeJA for the indicated periods. The total RNA was extracted from rosette leaves and analyzed by Northern hybridization.
Effect of MeJA on the Gene Expression of Arabidopsis CLHs.
Because, as described above, AtCLH1 already had been isolated as a coronatine- and MeJA-inducible gene in A. thaliana (27), we examined gene expression profiles of the Arabidopsis two CLHs by Northern analysis in the rosette leaves treated with MeJA. The AtCLH1 transcript was observed at approximately 1.3 kb, and that of AtCLH2 seemed to be at a slightly lower position. The expression of AtCLH1 mRNA was induced quickly after 10-μM MeJA treatment, reached a maximum at 3 h, and remained high for at least 9 h (Fig. 4B). In contrast, it is interesting that AtCLH2 mRNA constitutively expressed at a low level and did not respond to MeJA (Fig. 4B).
Discussion
In the present study, we isolated the gene involved in Chl degradation in higher plants. Our cloning of CLH genes is a significant breakthrough in the investigation of the molecular mechanism of Chl degradation, such as the physiological roles of CLH isozymes in vivo and the regulatory mechanism of these CLHs.
Because the CLH amino acid sequences contained a conserved lipase motif region, we aligned isolated CLH sequences with other known sequences of typical lipases, but the homology was less than 10%. We searched the CLH homologue in Synechocystis sp. PCC 6803, whose entire genomic sequence was determined (30); however, no sequence highly homologous to CLHs was found. In photosynthetic bacteria, we found a function-unknown gene (bchO, orf284) in the photosynthesis gene cluster of Rhodobacter capsulatus (31). Although the identity of BchO and CLHs was low in the sequence overall, a region was homologous to the lipase motif region of CLHs. Given that CLHs in higher plants share less than 40% homology with each other, this bacterial gene might be a CLH homologue in photosynthetic bacteria.
With respect to the difference in estimated molecular mass between the purified type 0 Chlase (42 kDa) and the recombinant Chlase (CaCLH, 36 kDa), we assume that the amino acid residue “X” in the AP-2 peptide in Fig. 1A is modified by glycosylation; thus, the molecular mass of the purified type 0 Chlase is greater than that of the recombinant Chlase (CaCLH). In fact, although we could not assign “X” to any amino acid residue by amino acid sequencing of type 0 Chlase, “X” was an Asn residue according to the deduced amino acid sequence. Because the sequence near the Asn residue had a typical glycosylation motif, N-{P}-[ST]-{P} (PS00001), it seemed likely that native CaCLH was modified by glycosylation at the Asn residue. This assumption agreed with this CaCLH having been purified with Con A Sepharose (21). Because Con A-interacting Chlase also exists in a diatom P. tricornutum (16, 17), the presence of the glycoprotein type Chlase might not be specific in C. album.
The amino acid sequence alignment between CaCLH and AtCLHs indicated that the N-terminal region exhibited much lower homology than other regions, suggesting that these enzymes had different signal peptides for transport. For AtCLHs, AtCLH2 has an N-terminal sequence enriched in hydroxylated amino acids and containing a few acidic amino acids, which is typical of the transit peptide sequence for chloroplast (32). AtCLH2 probably is in chloroplast, as is often reported (12, 13), although the localization of AtCLH1 is obscure. For CaCLH, we tried many times to isolate the gene encoding chloroplast-type CLH by the heterologous screening of a C. album cDNA library, but could not. However, because Chlase activity was observed in the lysate of intact chloroplasts isolated from C. album leaves (33), the chloroplast-type Chlase probably also exists in C. album.
The N-terminal region of CaCLH contains a positively charged amino acid (Lys-3) followed by a long, central, hydrophobic stretch and a more polar segment, a typical feature of the signal peptide for ER (34). The putative cleavage site of the signal peptide was predicted by the signalp program (35), and the underlined sequence at the N terminus in Fig. 1B seemed to be a signal sequence for ER. This result is also consistent with the above-described assumption that native CaCLH is modified by glycosylation at the Asn residue because ER is a major site for the glycosylation of proteins. Because there was no signal sequence for ER retention and 11 aa residues existed between the putative cleavage site in ER and the N terminus of the putative mature CaCLH, we assumed that CaCLH was transported further to other organelles and N-terminal propeptide was processed. In addition, the NPIR central motif, a vacuolar-sorting determinant conserved in prosporamin and proaleurain (26), also was found in the N-terminal region of CaCLH (dashed underline in Fig. 1B). Plant vacuole is well known to be a degradative compartment and contains various hydrolases (36), and the autophagic activity of vacuole is considered to play a central role in protein and organelle turnover (37, 38). Although phytol residue is considered to remain in senescing chloroplast (4), the most recent report suggests that Chl and/or its colored derivatives is secreted from senescing chloroplast to cytoplasm or vacuole (39). Moreover, most of the Chl catabolites were found in the vacuole fraction (40, 41). The circumstantial evidence described above leads us to speculate that CaCLH possibly is transported to vacuole via ER. If this speculation is true, then at least in C. album, another system of Chl degradation may exist outside of chloroplast besides the well known pathway in chloroplast (e.g., AtCLH2) where Chl degradation of early steps is considered to take place (4). In any case, the presence of the extraplastidic signal sequence is a very interesting finding, and further investigations such as those on the localization and import of CLHs in various plants are needed to solve these problems.
We found that one of the AtCLHs had been cloned as a gene whose mRNA is inducible by coronatine and MeJA (27). Coronatine is a chlorosis-inducing toxin produced by several pathovars of Pseudomonas syringae (42, 43). The chlorotic halo caused by the infection of these bacteria was observed. Thus, it is likely that the rapid induction of AtCLH1 mRNA is closely correlated with the loss of Chl during chlorosis. Because coronatine was known to act as a mimic of MeJA in plants (44), coi1 (coronatine-insensitive) mutant of A. thaliana is insensitive to coronatine and to MeJA (45). In fact, AtCLH1 (CORI1) mRNA was induced by both coronatine and MeJA, and its expression was repressed by coi1 mutation (27). Jasmonate and its derivative (MeJA) are plant growth regulators and have various effects on plants. Concerning these effects, it is interesting to note that MeJA promotes senescence of oat leaves (46) and Chl degradation in barley leaves (47). In fact, our preliminary experiment showed that the leaves of A. thaliana gradually became yellow on MeJA treatment under conditions similar to those reported for other plant species. Thus, it is reasonable that the mRNA of AtCLH1 involving Chl degradation is inducible by MeJA. Although the physiological significance of the rapid Chl breakdown is still unknown, it may be correlated with a rapid senescence of leaves caused by coronatine and jasmonate.
Acknowledgments
We thank Dr. Kazuo Shinozaki (The Institute of Physical and Chemical Research, Japan) for providing the cDNA library of A. thaliana. We also thank Professor Yuzo Shioi (Shizuoka University) for encouraging us during this study.
Abbreviations
- Chlase
chlorophyllase
- Chl
chlorophyll
- AtCLH
A. thaliana chlorophyllase gene
- CaCLH
C. album chlorophyllase gene
- MeJA
methyl jasmonate
- ER
endoplasmic reticulum
Footnotes
References
- 1.Hendry G A F, Houghton J D, Brown S B. New Phytol. 1987;107:255–302. doi: 10.1111/j.1469-8137.1987.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 2.Brown S B, Houghton J D, Hendry G A F. In: Chlorophylls. Scheer H, editor. Boca Raton, FL: CRC; 1991. pp. 465–489. [Google Scholar]
- 3.Thomas H. New Phytol. 1997;136:163–181. [Google Scholar]
- 4.Matile P, Hörtensteiner S, Thomas H. In: Annu. Rev. Plant Physiol. Plant Mol. Biol. Jones R L, Bohnert H J, Walbot V, editors. Palo Alto, CA: Annual Reviews; 1999. pp. 67–95. [DOI] [PubMed] [Google Scholar]
- 5.Shioi Y, Tatsumi Y, Shimokawa K. Plant Cell Physiol. 1991;32:87–93. [Google Scholar]
- 6.Willstätter R, Stoll A, editors. Untersuchungen über Chlorophyll. Berlin: Springer; 1913. pp. 172–187. [Google Scholar]
- 7.Drazkiewicz M. Photosynthetica. 1994;30:321–331. [Google Scholar]
- 8.Trebitsh T, Goldschmidt E E, Riov J. Proc Natl Acad Sci USA. 1993;90:9441–9445. doi: 10.1073/pnas.90.20.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamai H, Shioi Y, Sasa T. Plant Cell Physiol. 1979;20:1141–1145. [Google Scholar]
- 10.Khalyfa A, Kermasha S, Marsot P, Goetghebeur M. Appl Biochem Biotechnol. 1995;53:11–27. [Google Scholar]
- 11.Shimokawa K. Phytochemistry. 1982;21:543–545. [Google Scholar]
- 12.Terpstra W. Z Pflanzenphysiol. 1974;71:129–143. [Google Scholar]
- 13.Tarasenko L G, Khodasevich E V, Orlovskaya K I. Photobiochem Photobiophys. 1986;12:119–121. [Google Scholar]
- 14.Brandis A, Vainstein A, Goldschmidt E E. Plant Physiol Biochem. 1996;34:49–54. [Google Scholar]
- 15.Matile P, Schellenberg M, Vicentini F. Planta. 1997;201:96–99. [Google Scholar]
- 16.Terpstra W. FEBS Lett. 1981;126:231–235. [Google Scholar]
- 17.Terpstra W, Lambers J W J, Levine Y K. Photobiochem Photobiophys. 1986;11:249–255. [Google Scholar]
- 18.Shioi Y, Tomita N, Tsuchiya T, Takamiya K. Plant Physiol Biochem. 1996;34:41–47. [Google Scholar]
- 19.Shioi Y, Watanabe K, Takamiya K. Plant Cell Physiol. 1996;37:1143–1149. doi: 10.1093/oxfordjournals.pcp.a028926. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe K, Ohta H, Tsuchiya T, Mikami B, Masuda T, Shioi Y, Takamiya K. Plant Cell Physiol. 1999;40:104–108. [Google Scholar]
- 21.Tsuchiya T, Ohta H, Masuda T, Mikami B, Kita N, Shioi Y, Takamiya K. Plant Cell Physiol. 1997;38:1026–1031. [Google Scholar]
- 22.Iwamatsu A. Electrophoresis. 1992;13:142–147. doi: 10.1002/elps.1150130129. [DOI] [PubMed] [Google Scholar]
- 23.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Chory J. In: Methods in Molecular Biology. Martinez-Zapater J, Salinas J, editors. Totowa, NJ: Humana; 1998. pp. 55–60. [Google Scholar]
- 25.Murashige T, Skoog F. Physiol Plant. 1962;15:473–497. [Google Scholar]
- 26.Neuhaus J-M, Rogers J C. Plant Mol Biol. 1998;38:127–144. [PubMed] [Google Scholar]
- 27.Benedetti C E, Costa C L, Turcinelli S R, Arruda P. Plant Physiol. 1998;116:1037–1042. doi: 10.1104/pp.116.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathur J, Szabados L, Schaefer S, Grunenberg B, Lossow A, Jonas-Straube E, Schell J, Koncz C, Koncz-Kálmán Z. Plant J. 1998;13:707–716. doi: 10.1046/j.1365-313x.1998.00059.x. [DOI] [PubMed] [Google Scholar]
- 29.Bairoch A. Nucleic Acids Res. 1991;19:2241–2245. doi: 10.1093/nar/19.suppl.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, et al. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 31.Bollivar D W, Suzuki J Y, Beatty J T, Dobrowolski J M, Bauer C E. J Mol Biol. 1994;237:622–640. doi: 10.1006/jmbi.1994.1260. [DOI] [PubMed] [Google Scholar]
- 32.von Heijne G, Steppuhn J, Herrmann R G. Eur J Biochem. 1989;180:535–545. doi: 10.1111/j.1432-1033.1989.tb14679.x. [DOI] [PubMed] [Google Scholar]
- 33.Kita N, Takamiya K, Shioi Y. In: Research in Photosynthesis. Murata N, editor. Dordrecht, The Netherlands: Kluwer; 1992. pp. 115–118. [Google Scholar]
- 34.von Heijne G, Abrahmsén L. FEBS Lett. 1989;244:439–446. doi: 10.1016/0014-5793(89)80579-4. [DOI] [PubMed] [Google Scholar]
- 35.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Nishimura M, Beevers H. Plant Physiol. 1978;62:44–48. doi: 10.1104/pp.62.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marty F. Plant Cell. 1999;11:587–599. doi: 10.1105/tpc.11.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aubert S, Gout E, Bligny R, Marty-Mazars D, Barrieu F, Alabouvette J, Marty F, Douce R. J Cell Biol. 1996;133:1251–1263. doi: 10.1083/jcb.133.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guiamét J J, Pichersky E, Noodén L D. Plant Cell Physiol. 1999;40:986–992. [Google Scholar]
- 40.Düggelin T, Schellenberg M, Bortlik K, Matile P. J Plant Physiol. 1988;133:492–497. [Google Scholar]
- 41.Matile P, Ginsburg S, Schellenberg M, Thomas H. Proc Natl Acad Sci USA. 1988;85:9529–9532. doi: 10.1073/pnas.85.24.9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell R E, Young H. Phytochemistry. 1978;17:2028–2029. [Google Scholar]
- 43.Mitchell R E, Hale C N, Shanks J C. Physiol Plant Pathol. 1983;23:315–322. [Google Scholar]
- 44.Weiler E W, Kutchan T M, Gorba T, Brodschelm W, Niesel U, Bublitz F. FEBS Lett. 1994;345:9–13. doi: 10.1016/0014-5793(94)00411-0. [DOI] [PubMed] [Google Scholar]
- 45.Feys B J, Benedetti C E, Penfold C N, Turner J G. Plant Cell. 1994;6:751–759. doi: 10.1105/tpc.6.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ueda J, Kato J. Plant Physiol. 1980;66:246–249. doi: 10.1104/pp.66.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weidhase R A, Lehmann J, Kramell H, Sembdner G, Parthier B. Physiol Plant. 1987;69:161–166. [Google Scholar]