Abstract
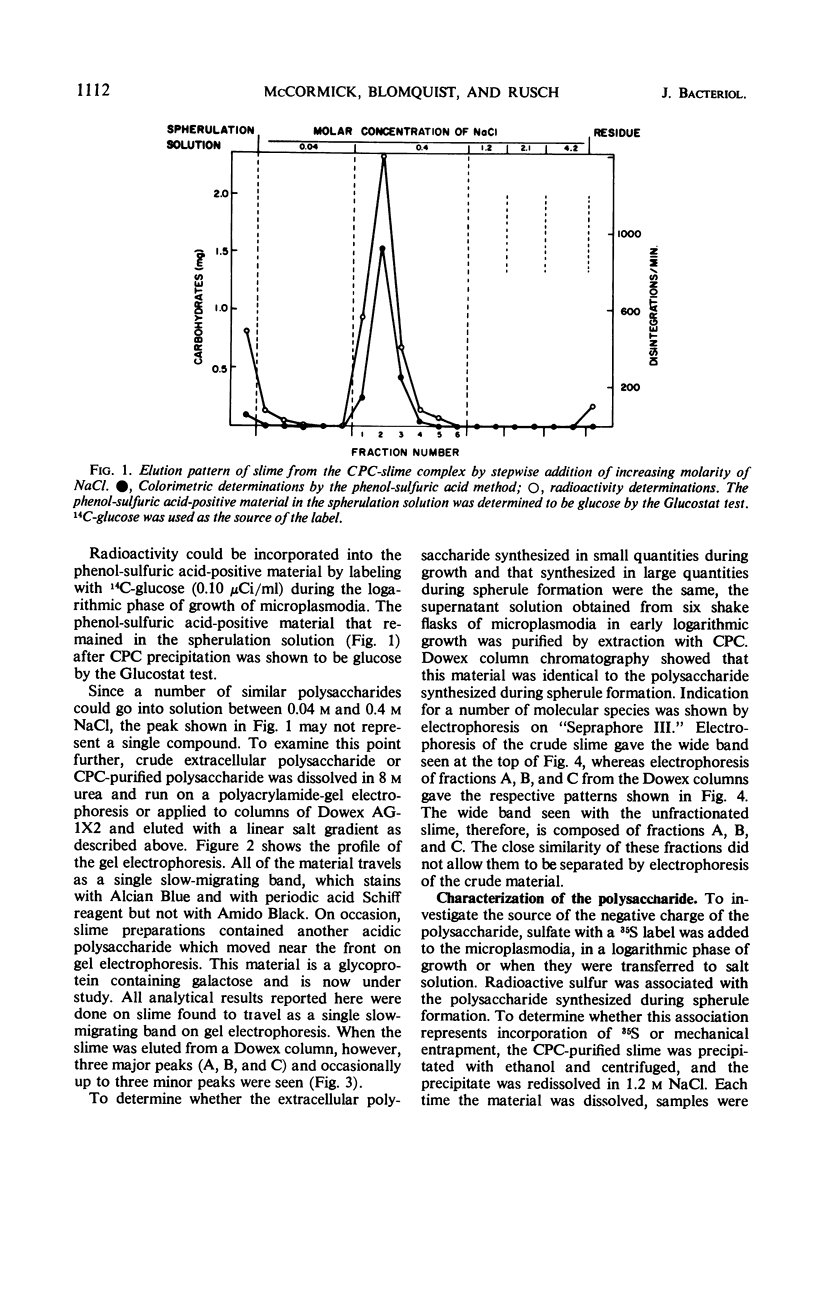
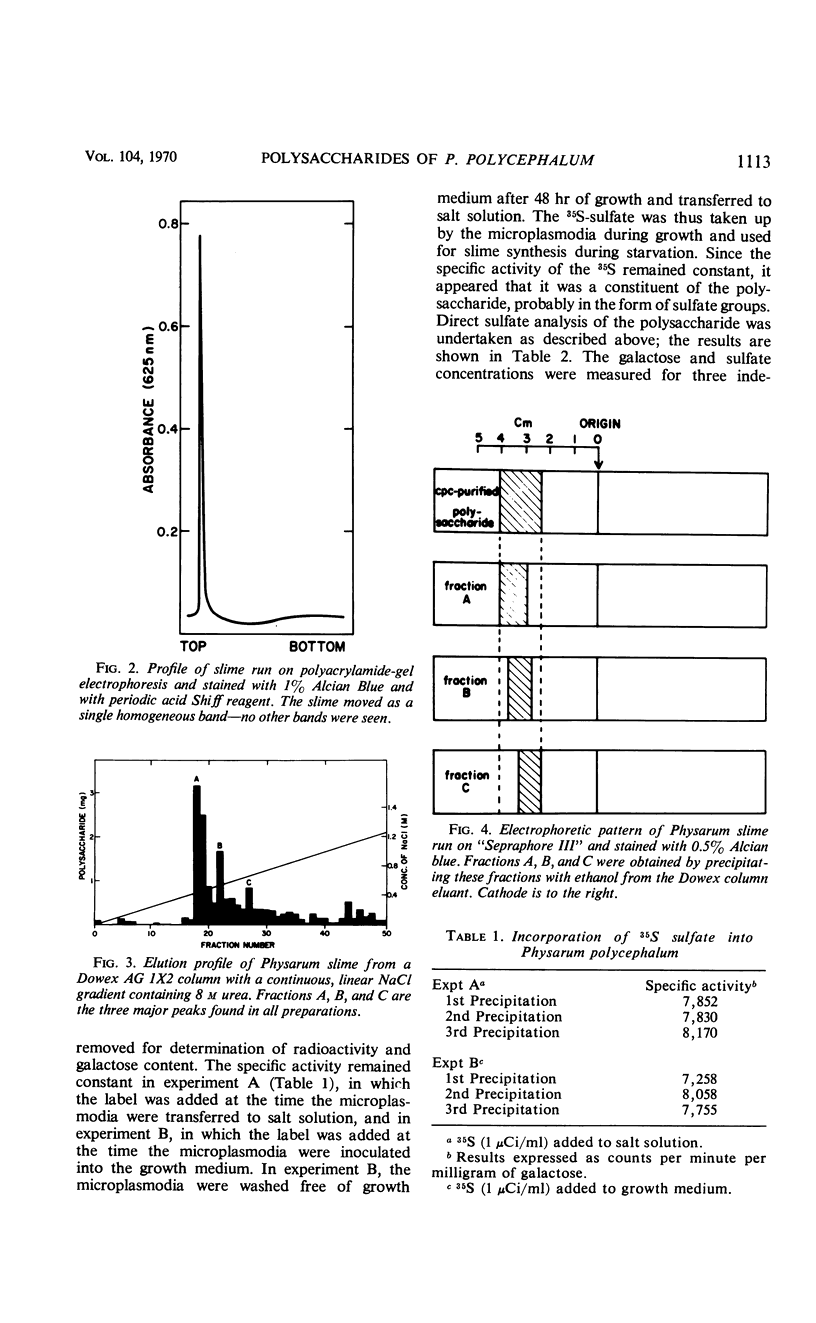
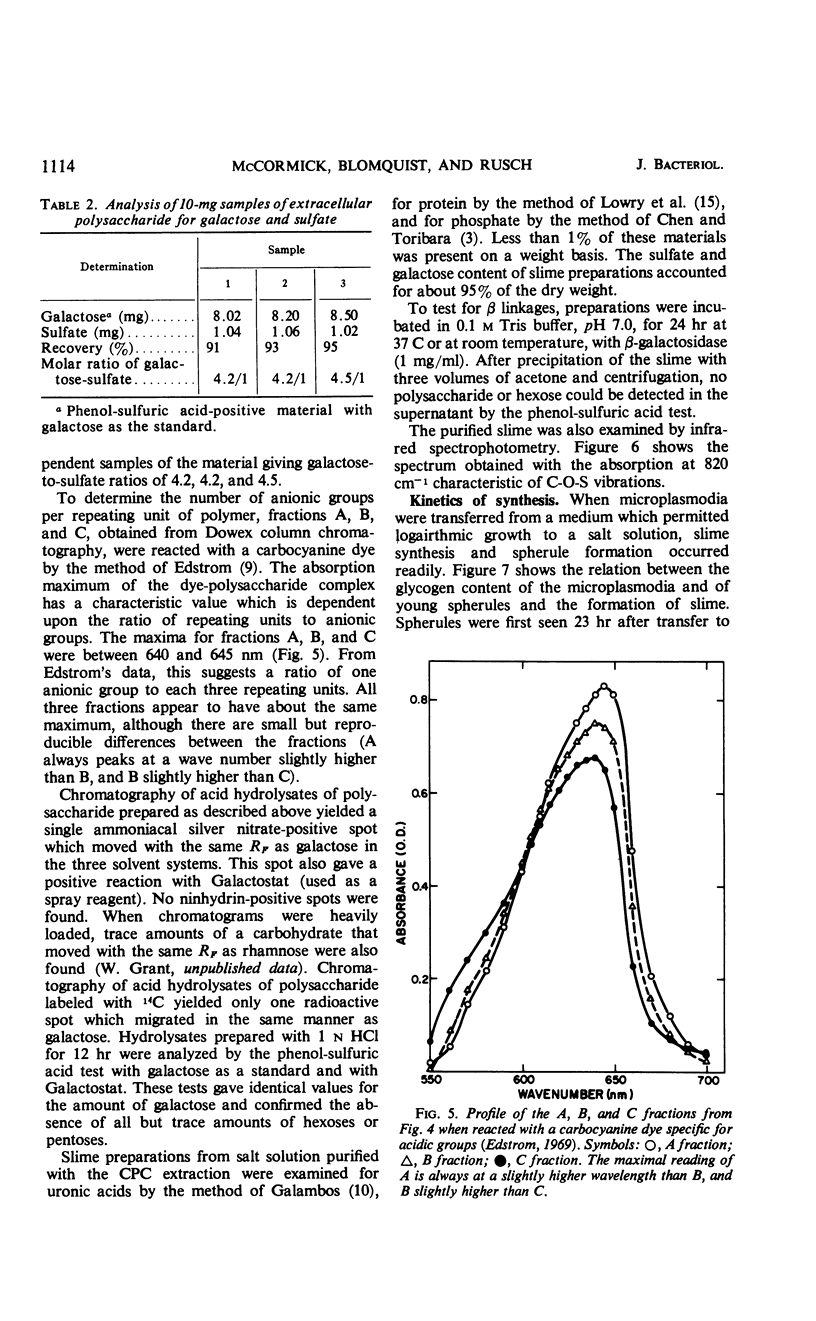
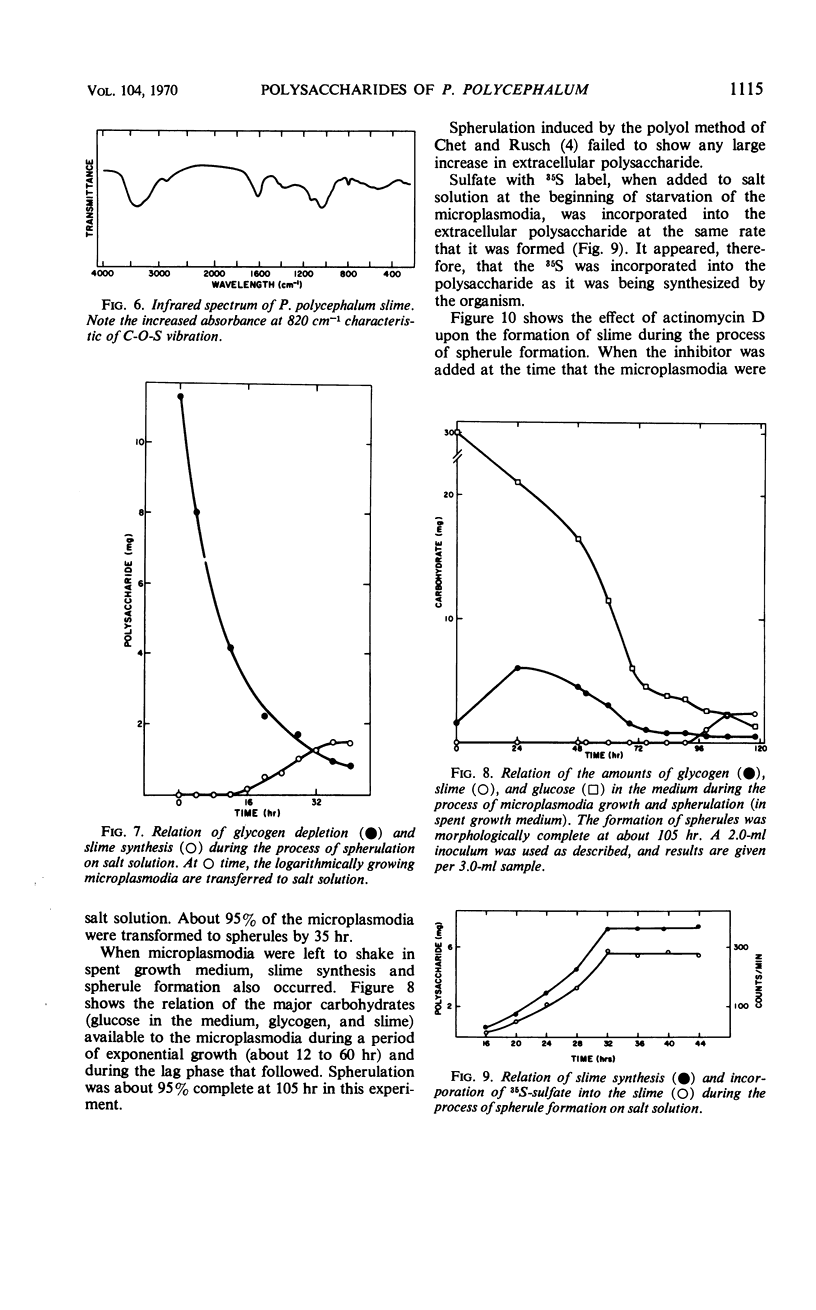
The myxomycetes are called slime molds because of the synthesis of copious amounts of extracellular material (slime) during parts of the life cycle. In Physarum polycephalum, small amounts of slime are produced during exponential growth of microplasmodia in shake flasks, but the amount of this slime increased 10- to 20-fold at 16 to 34 hr after microplasmodia were induced to form spherules by transferring them to salt solution. The slime obtained during both periods is the same; an acidic polysaccharide consisting of galactose, sulfate, and trace amounts of rhamnose. Analysis of the galactose-to-sulfate ratio gave a value of about 4 to 1. Infrared spectroscopy showed increased absorbance at 820 cm−1 characteristic of C-O-S vibrations. Electrophoresis on polyacrylamide gel revealed that the material moved as a single band which stained with Alcian Blue and periodic acid Shiff reagent. However, fractionation of identical material on Dowex columns and electrophoresis on cellulose acetate showed the slime to be made up of three major fractions. The polysaccharide appeared as an extracellular capsule closely adhering to the walls of the spherules. It could be separated from the wall by vigorous shaking. The increased synthesis of slime during spherulation was not blocked by cycloheximide, suggesting that new enzyme synthesis was not necessary for its formation.
Full text
PDF

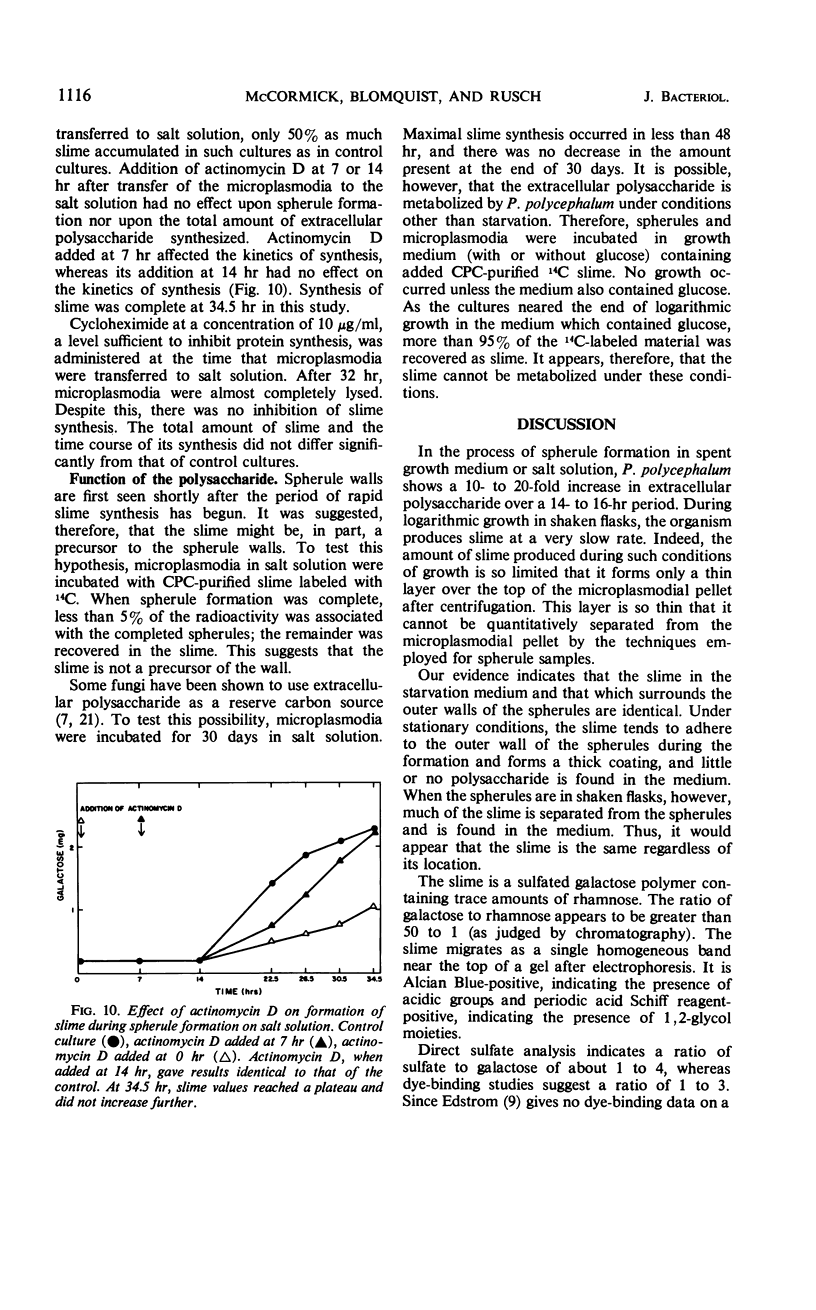


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. S., Dolan T. C., Rees D. A. Evidence for a common structural pattern in the polysaccharide sulphates of the Rhodophyceae. Nature. 1965 Mar 13;205(976):1060–1062. doi: 10.1038/2051060a0. [DOI] [PubMed] [Google Scholar]
- CALDWELL R. C., PIGMAN W. DISC ELECTROPHORESIS OF HUMAN SALIVA IN POLYACRYLAMIDE GEL. Arch Biochem Biophys. 1965 Apr;110:91–96. doi: 10.1016/0003-9861(65)90158-x. [DOI] [PubMed] [Google Scholar]
- Chet I., Rusch H. P. Induction of spherule formation in Physarum polycephalum by polyols. J Bacteriol. 1969 Nov;100(2):673–678. doi: 10.1128/jb.100.2.673-678.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DAVIS E. N., RHODES R. A., SHULKE H. R. FERMENTATIVE PRODUCTION OF EXOCELLULAR GLUCANS BY FLESHY FUNGI. Appl Microbiol. 1965 Mar;13:267–271. doi: 10.1128/am.13.2.267-271.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edstrom R. D. A colorimetric method for the determination of mucopolysaccharides and other acidic polymers. Anal Biochem. 1969 Jun;29(3):421–432. doi: 10.1016/0003-2697(69)90327-3. [DOI] [PubMed] [Google Scholar]
- Galambos J. T. The reaction of carbazole with carbohydrates. I. Effect of borate and sulfamate on the carbazole color of sugars. Anal Biochem. 1967 Apr;19(1):119–132. doi: 10.1016/0003-2697(67)90141-8. [DOI] [PubMed] [Google Scholar]
- Goodman E. M., Sauer H. W., Sauer L., Rusch H. P. Polyphosphate and other phosphorus compounds during growth and differentiation of Physarum polycephalum. Can J Microbiol. 1969 Nov;15(11):1325–1331. doi: 10.1139/m69-240. [DOI] [PubMed] [Google Scholar]
- KRISMAN C. R. A method for the colorimetric estimation of glycogen with iodine. Anal Biochem. 1962 Jul;4:17–23. doi: 10.1016/0003-2697(62)90014-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pearce R. H., Mathieson J. M., Grimmer B. J. Fractionation of anionic glycosaminoglycans by ion-exchange chromatography. Anal Biochem. 1968 Jul;24(1):141–156. doi: 10.1016/0003-2697(68)90069-9. [DOI] [PubMed] [Google Scholar]
- SCHILLER S., SLOVER G. A., DORFMAN A. A method for the separation of acid mucopolysaccharides: its application to the isolation of heparin from the skin of rats. J Biol Chem. 1961 Apr;236:983–987. [PubMed] [Google Scholar]
- SCOTT J. E. Aliphatic ammonium salts in the assay of acidic polysaccharides from tissues. Methods Biochem Anal. 1960;8:145–197. doi: 10.1002/9780470110249.ch4. [DOI] [PubMed] [Google Scholar]
- Simon H. L., Henney H. R. Chemical composition of slime from three species of myxomycetes. FEBS Lett. 1970 Mar 16;7(1):80–82. doi: 10.1016/0014-5793(70)80623-8. [DOI] [PubMed] [Google Scholar]
- Szaniszlo P. J., Wirsen C., Jr, Mitchell R. Production of a capsular polysaccharide by a marine filamentous fungus. J Bacteriol. 1968 Nov;96(5):1474–1483. doi: 10.1128/jb.96.5.1474-1483.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Handel E. Estimation of glycogen in small amounts of tissue. Anal Biochem. 1965 May;11(2):256–265. doi: 10.1016/0003-2697(65)90013-8. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]


