Abstract
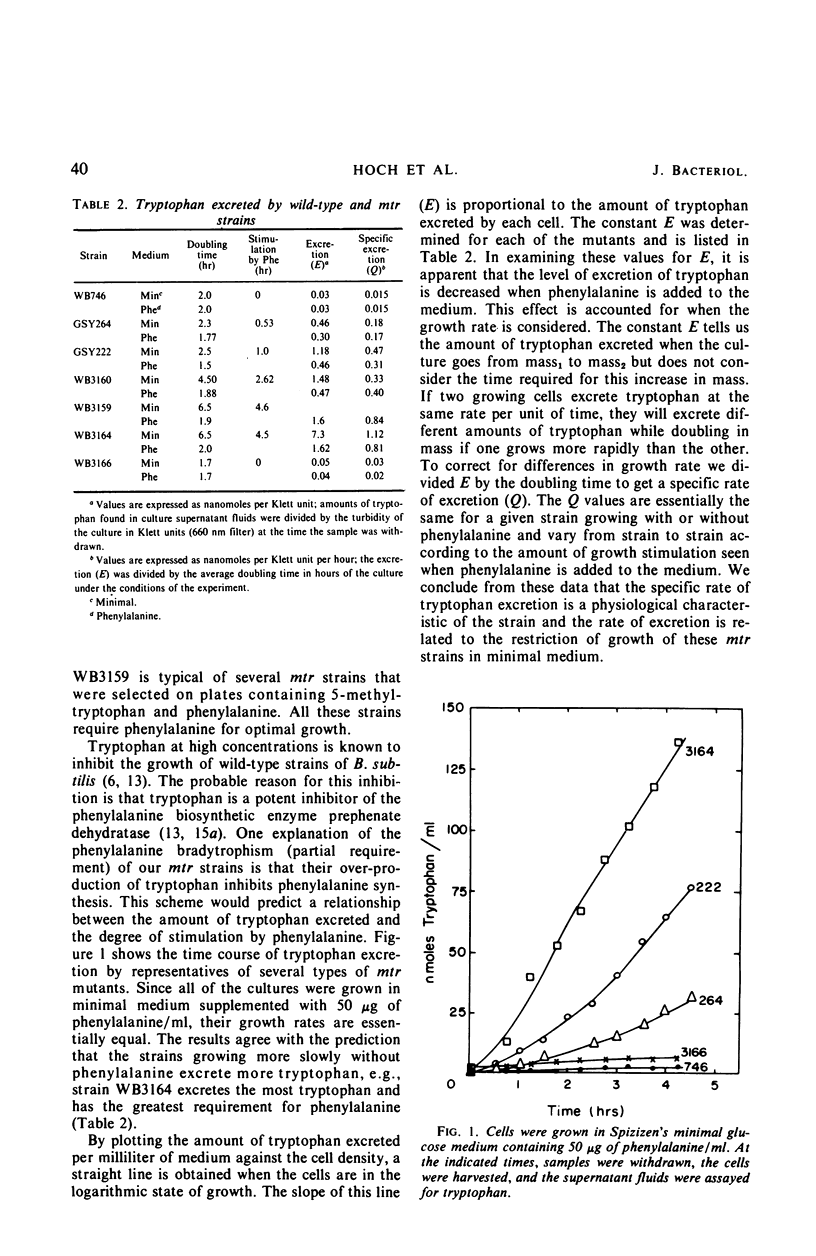
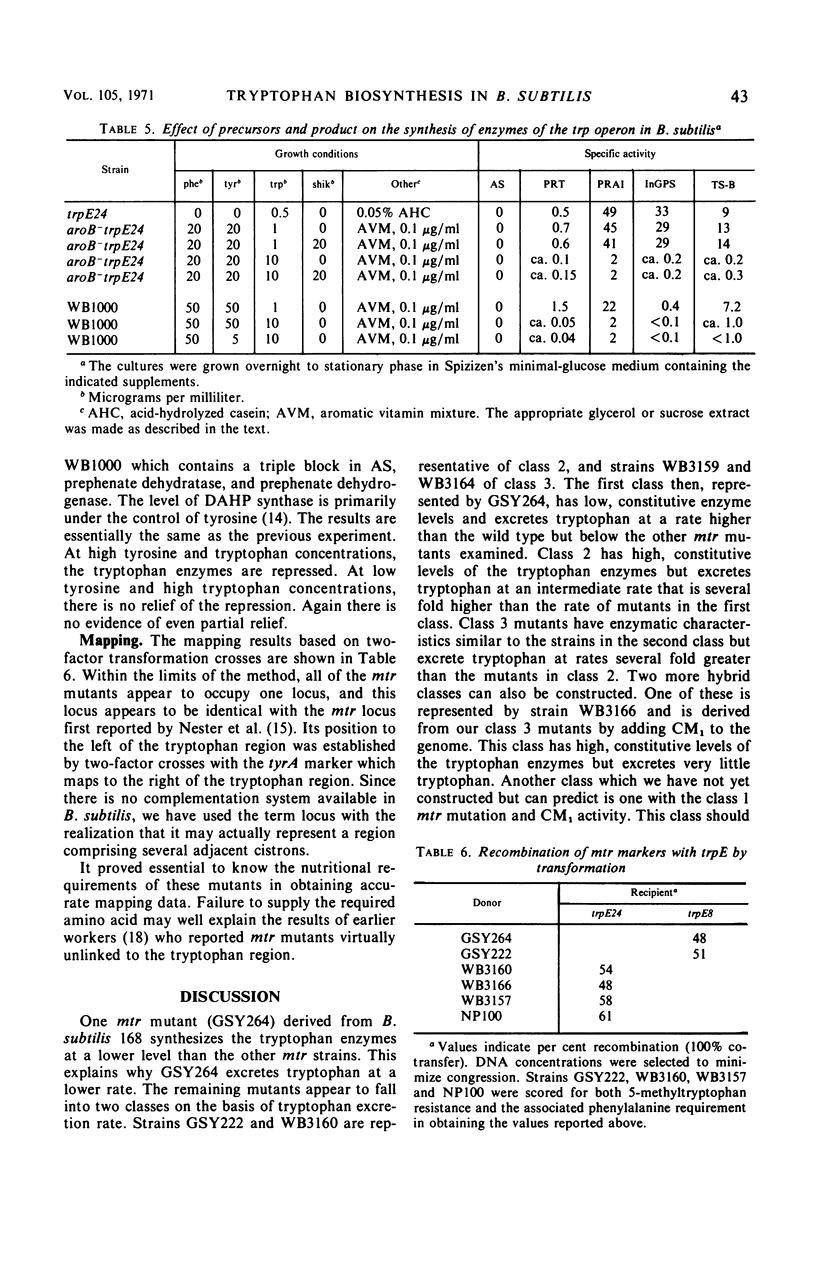
5-Methyltryptophan-resistant mutants derived from Bacillus subtilis strain 168 synthesize all of the tryptophan biosynthetic enzymes constitutively and excrete tryptophan. These mutants can be divided into three classes: class 1, low enzyme level and low rate of tryptophan excretion; class 2, high enzyme level and intermediate rate of tryptophan excretion; and class 3, high enzyme level and high rate of tryptophan excretion. A bradytrophic requirement for phenylalanine is correlated with the rate of tryptophan excretion. The phenylalanine requirement is relieved when the rate of tryptophan excretion is reduced by either (i) lowering the level of the tryptophan enzymes, (ii) reducing the supply of a tryptophan precursor (chorismate), or (iii) stopping tryptophan synthesis by a mutational block in the pathway. All of the mutants map in a region of the chromosome previously reported as the mtr locus. Our data show that synthesis of the tryptophan enzymes is controlled through the mtr locus but not influenced by precursors of tryptophan.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANAGNOSTOPOULOS C., CRAWFORD I. P. Transformation studies on the linkage of markers in the tryptophan pathway in Bacillus subtilis. Proc Natl Acad Sci U S A. 1961 Mar 15;47:378–390. doi: 10.1073/pnas.47.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G., JACOB F. Sur la répression de la synthèse des enzymes intervenant dans la formation du tryptophane chez Escherichia coll. C R Hebd Seances Acad Sci. 1959 Jun 15;248(24):3490–3492. [PubMed] [Google Scholar]
- FANGMAN W. L., NEIDHARDT F. C. DEMONSTRATION OF AN ALTERED AMINOACYL RIBONUCLEIC ACID SYNTHETASE IN A MUTANT OF ESCHERICHIA COLI. J Biol Chem. 1964 Jun;239:1839–1843. [PubMed] [Google Scholar]
- Hiraga S. Operator mutants of the tryptophan operon in Escherichia coli. J Mol Biol. 1969 Jan 14;39(1):159–179. doi: 10.1016/0022-2836(69)90340-4. [DOI] [PubMed] [Google Scholar]
- Hoch S. O., Anagnostopoulos C., Crawford I. P. Enzymes of the tryptophan operon of Bacillus subtilis. Biochem Biophys Res Commun. 1969 Jun 27;35(6):838–844. doi: 10.1016/0006-291x(69)90700-1. [DOI] [PubMed] [Google Scholar]
- Jensen R. A. Metabolic interlock. Regulatory interactions exerted between biochemical pathways. J Biol Chem. 1969 Jun 10;244(11):2816–2823. [PubMed] [Google Scholar]
- Kane J. F., Jensen R. A. Enzyme induction in the tryptophan biosynthetic pathway in Bacillus subtilis. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1161–1167. doi: 10.1016/0006-291x(70)90361-x. [DOI] [PubMed] [Google Scholar]
- Kane J. F., Jensen R. A. Metabolic interlock. The influence of histidine on tryptophan biosynthesis in Bacillus subtilis. J Biol Chem. 1970 May 10;245(9):2384–2390. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lorence J. H., Nester E. W. Multiple molecular forms of chorismate mutase in Bacillus subtillis. Biochemistry. 1967 May;6(5):1541–1553. doi: 10.1021/bi00857a041. [DOI] [PubMed] [Google Scholar]
- MOYED H. S. False feedback inhibition: inhibition of tryptophan biosynthesis by 5-methyltryptophan. J Biol Chem. 1960 Apr;235:1098–1102. [PubMed] [Google Scholar]
- Morse D. E., Yanofsky C. Amber mutants of the trpR regulatory gene. J Mol Biol. 1969 Aug 28;44(1):185–193. doi: 10.1016/0022-2836(69)90413-6. [DOI] [PubMed] [Google Scholar]
- Nester E W, Schafer M, Lederberg J. Gene Linkage in DNA Transfer: A Cluster of Genes Concerned with Aromatic Biosynthesis in Bacillus Subtilis. Genetics. 1963 Apr;48(4):529–551. doi: 10.1093/genetics/48.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E. W., Jensen R. A. Control of aromatic acid biosynthesis in Bacillus subtilis: sequenial feedback inhibition. J Bacteriol. 1966 Apr;91(4):1594–1598. doi: 10.1128/jb.91.4.1594-1598.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E. W., Jensen R. A., Nasser D. S. Regulation of enzyme synthesis in the aromatic amino acid pathway of Bacillus subtilus. J Bacteriol. 1969 Jan;97(1):83–90. doi: 10.1128/jb.97.1.83-90.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebello J. L., Jensen R. A. Metabolic interlock. The multi-metabolite control of prephenate dehydratase activity in Bacillus subtilis. J Biol Chem. 1970 Aug 10;245(15):3738–3744. [PubMed] [Google Scholar]
- SOMERVILLE R. L., YANOFSKY C. STUDIES ON THE REGULATION OF TRYPTOPHAN BIOSYNTHESIS IN ESCHERICHIA COLI. J Mol Biol. 1965 Apr;11:747–759. doi: 10.1016/s0022-2836(65)80032-8. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitt D. D., Carlton B. C. Non-coordinate regulation in 5-methyl tryptophan-resistant mutants of Bacillus subtilis. Biochem Biophys Res Commun. 1968 Nov 25;33(4):636–642. doi: 10.1016/0006-291x(68)90343-4. [DOI] [PubMed] [Google Scholar]


