Abstract
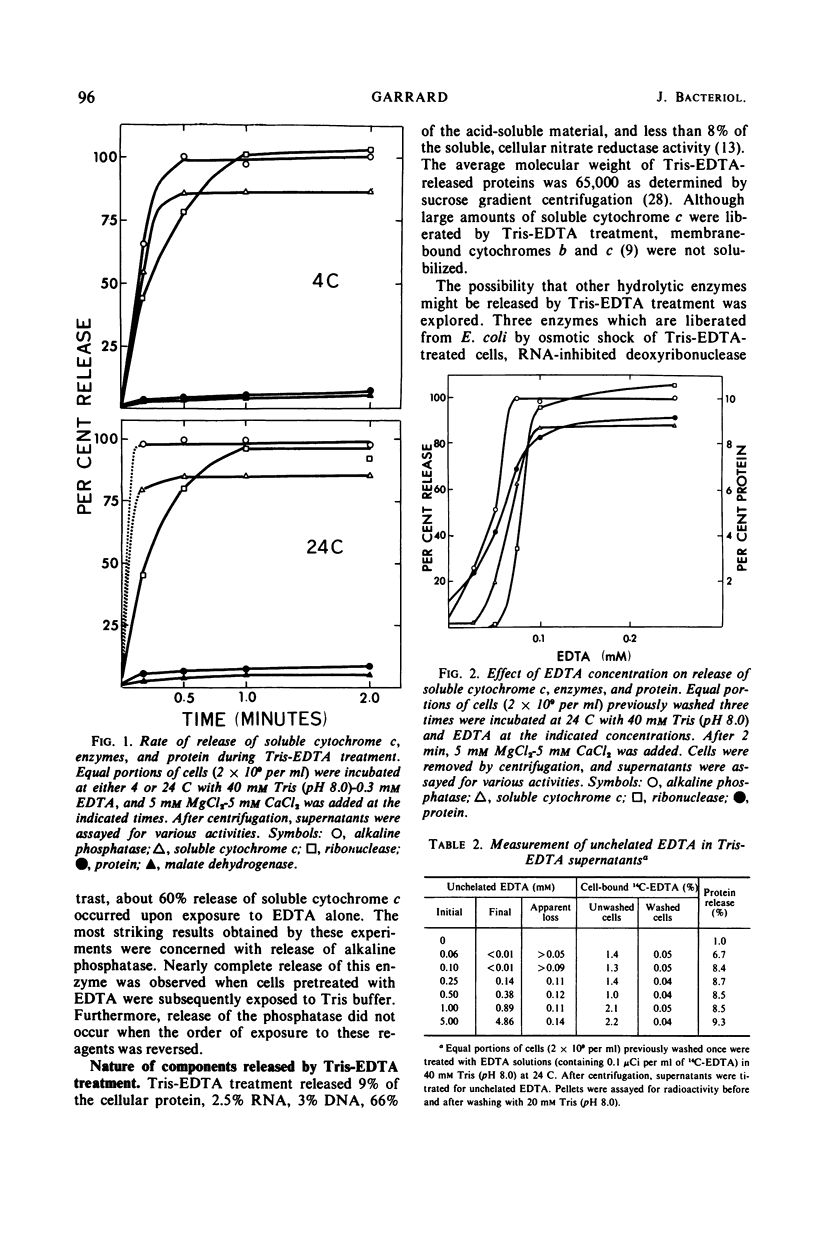
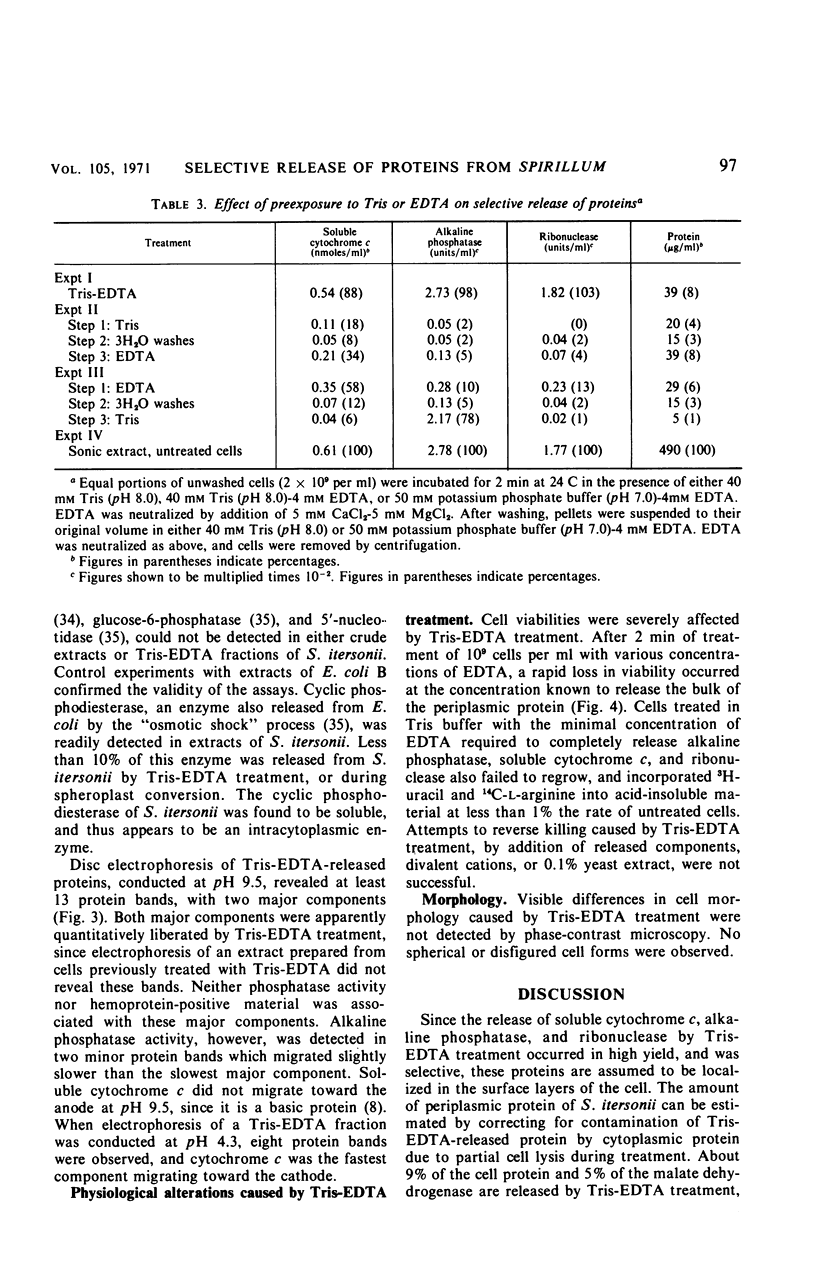
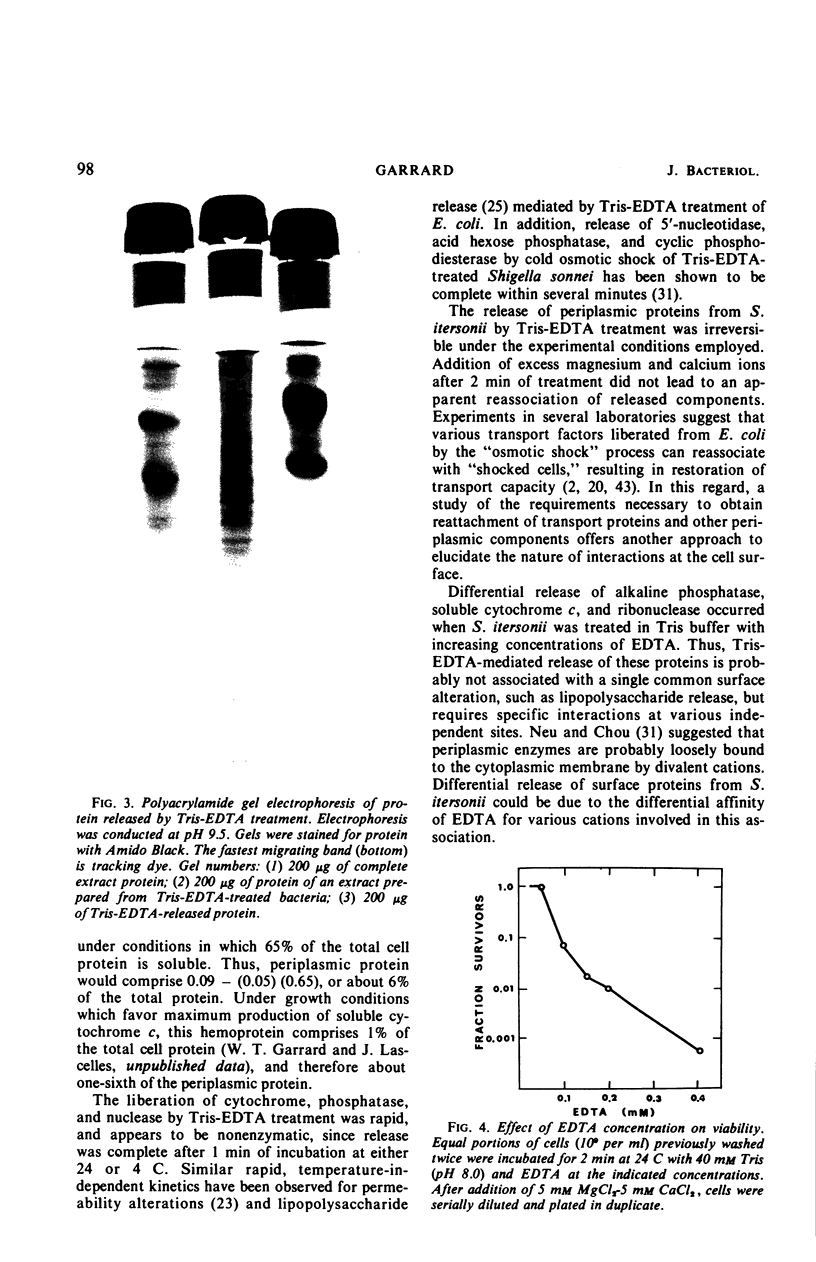
Treatment of Spirillum itersonii with tris(hydroxymethyl)aminomethane (Tris)-ethylenediaminetetraacetate (EDTA) results in the quantitative release of alkaline phosphatase and ribonuclease into the surrounding medium. At the same time, about 90% of the total cellular soluble cytochrome c is liberated. This process occurs within 1 min of treatment at both 24 and 4 C. Release of these proteins by Tris-EDTA treatment is highly selective, since only 9% of the total cell protein is liberated, concomitantly with less than 5% ribonucleic acid, deoxyribonucleic acid, and malate dehydrogenase. Different sigmoidal curves are obtained for release of proteins as a function of EDTA concentration. The order of liberation with increasing EDTA is as follows: alkaline phosphatase, protein, soluble cytochrome c, and ribonuclease. Treatment of cells with Tris-EDTA under conditions which cause extensive loss of alkaline phosphatase, soluble cytochrome c, and ribonuclease results in cell death, with cessation of protein and ribonucleic acid synthesis. Cells treated with EDTA in phosphate buffer (in the absence of Tris) liberate a large portion of their soluble cytochrome c, but negligible amounts of alkaline phosphatase and ribonuclease. Addition of Tris to cells pretreated with phosphate-buffered EDTA releases high levels of alkaline phosphatase, but not ribonuclease. These results suggest that a common surface alteration is not solely responsible for release of periplasmic proteins. More likely, each protein of the periplasm is bound in an independent and specific manner.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anraku Y., Heppel L. A. On the nature of the changes induced in Escherichia coli by osmotic shock. J Biol Chem. 1967 May 25;242(10):2561–2569. [PubMed] [Google Scholar]
- Anraku Y. The reduction and restoration of galactose transport in osmotically shocked cells of Escherichia coli. J Biol Chem. 1967 Mar 10;242(5):793–800. [PubMed] [Google Scholar]
- Anraku Y. Transport of sugars and amino acids in bacteria. I. Purification and specificity of the galactose- and leucine-binding proteins. J Biol Chem. 1968 Jun 10;243(11):3116–3122. [PubMed] [Google Scholar]
- Asbell M. A., Eagon R. G. Role of Multivalent Cations in the Organization, Structure, and Assembly of the Cell Wall of Pseudomonas aeruginosa. J Bacteriol. 1966 Aug;92(2):380–387. doi: 10.1128/jb.92.2.380-387.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman R. W., Heppel L. A. On the localization of alkaline phosphatase and cyclic phosphodiesterase in Escherichia coli. Biochemistry. 1968 Jul;7(7):2554–2562. doi: 10.1021/bi00847a016. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D., Lascelles J. Cytochrome c550 from Spirillum itersonii: purification and some properties. Arch Biochem Biophys. 1970 Jan;136(1):153–159. doi: 10.1016/0003-9861(70)90336-x. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D., Rittenberg B., Lascelles J. Cytochrome synthesis and its regulation in Spirillum itersonii. J Bacteriol. 1967 Nov;94(5):1648–1655. doi: 10.1128/jb.94.5.1648-1655.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F. Metallo-enzymes released from Escherichia coli by osmotic shock. I. Selective depression of enzymes in cells grown in the presence of ethylenediaminetetraacetate. J Biol Chem. 1968 May 25;243(10):2640–2646. [PubMed] [Google Scholar]
- Gauthier D. K., Clark-Walker G. D., Garrard W. T., Jr, Lascelles J. Nitrate reductase and soluble cytochrome c in Spirillum itersonii. J Bacteriol. 1970 Jun;102(3):790–801. doi: 10.1128/jb.102.3.797-803.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt M. C., Wyss O. The role of tris in EDTA toxicity and lysozyme lysis. J Gen Microbiol. 1967 Jun;47(3):421–431. doi: 10.1099/00221287-47-3-421. [DOI] [PubMed] [Google Scholar]
- HUMPHREY B., VINCENT J. M. Calcium in cell walls of Rhizobium trifolii. J Gen Microbiol. 1962 Nov;29:557–561. doi: 10.1099/00221287-29-3-557. [DOI] [PubMed] [Google Scholar]
- Hanlon D. P., Watt D. S., Westhead E. W. The interaction of divalent metal ions with tris buffer in dilute solution. Anal Biochem. 1966 Aug;16(2):225–233. doi: 10.1016/0003-2697(66)90150-3. [DOI] [PubMed] [Google Scholar]
- Heppel L. A. Selective release of enzymes from bacteria. Science. 1967 Jun 16;156(3781):1451–1455. doi: 10.1126/science.156.3781.1451. [DOI] [PubMed] [Google Scholar]
- Hjertén S., Jerstedt S., Tiselius A. Some aspects of the use of "continuous" and "discontinuous" buffer systems in polyacrylamide gel electrophoresis. Anal Biochem. 1965 May;11(2):219–223. doi: 10.1016/0003-2697(65)90008-4. [DOI] [PubMed] [Google Scholar]
- Kundig W., Kundig F. D., Anderson B., Roseman S. Restoration of active transport of glycosides in Escherichia coli by a component of a phosphotransferase system. J Biol Chem. 1966 Jul 10;241(13):3243–3246. [PubMed] [Google Scholar]
- LEIVE L. A NONSPECIFIC INCREASE IN PERMEABILITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Proc Natl Acad Sci U S A. 1965 Apr;53:745–750. doi: 10.1073/pnas.53.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leive L., Kollin V. Controlling EDTA treatment to produce permeable Escherichia coli with normal metabolic processes. Biochem Biophys Res Commun. 1967 Jul 21;28(2):229–236. doi: 10.1016/0006-291x(67)90434-2. [DOI] [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Leive L., Shovlin V. K., Mergenhagen S. E. Physical, chemical, and immunological properties of lipopolysaccharide released from Escherichia coli by ethylenediaminetetraacetate. J Biol Chem. 1968 Dec 25;243(24):6384–6391. [PubMed] [Google Scholar]
- Leive L. Studies on the permeability change produced in coliform bacteria by ethylenediaminetetraacetate. J Biol Chem. 1968 May 10;243(9):2373–2380. [PubMed] [Google Scholar]
- MALAMY M., HORECKER B. L. The localization of alkaline phosphatase in E. coli K12. Biochem Biophys Res Commun. 1961 Jun 2;5:104–108. doi: 10.1016/0006-291x(61)90020-1. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- NEU H. C., HEPPEL L. A. THE RELEASE OF RIBONUCLEASE INTO THE MEDIUM WHEN ESCHERICHIA COLI CELLS ARE CONVERTED TO SPEROPLASTS. J Biol Chem. 1964 Nov;239:3893–3900. [PubMed] [Google Scholar]
- Neu H. C., Chou J. Release of surface enzymes in Enterobacteriaceae by osmotic shock. J Bacteriol. 1967 Dec;94(6):1934–1945. doi: 10.1128/jb.94.6.1934-1945.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. On the surface localization of enzymes in E. coli. Biochem Biophys Res Commun. 1964 Oct 14;17(3):215–219. doi: 10.1016/0006-291x(64)90386-9. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of ribonuclease into the medium when E. coli cells are converted to spheroplasts. Biochem Biophys Res Commun. 1964;14:109–112. doi: 10.1016/0006-291x(64)90238-4. [DOI] [PubMed] [Google Scholar]
- Neu H. C. The role of amine buffers in EDTA toxicity and their effect on osmotic shock. J Gen Microbiol. 1969 Aug;57(2):215–220. doi: 10.1099/00221287-57-2-215. [DOI] [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- Pardee A. B., Prestidge L. S., Whipple M. B., Dreyfuss J. A binding site for sulfate and its relation to sulfate transport into Salmonella typhimurium. J Biol Chem. 1966 Sep 10;241(17):3962–3969. [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino-acid-binding protein released from Escherichia coli by osmotic shock. J Biol Chem. 1966 Dec 10;241(23):5732–5734. [PubMed] [Google Scholar]
- Sherman F., Stewart J. W., Parker J. H., Inhaber E., Shipman N. A., Putterman G. J., Gardisky R. L., Margoliash E. The mutational alteration of the primary structure of yeast iso-1-cytochrome c. J Biol Chem. 1968 Oct 25;243(20):5446–5456. [PubMed] [Google Scholar]
- Sumner J. B. A METHOD FOR THE COLORIMETRIC DETERMINATION OF PHOSPHORUS. Science. 1944 Nov 3;100(2601):413–414. doi: 10.1126/science.100.2601.413. [DOI] [PubMed] [Google Scholar]
- Tucker A. N., White D. C. Release of membrane components from viable Haemophilus parainfluenzae by ethylenediaminetetraacetic acid-tris(hydroxymethyl)-aminomethane. J Bacteriol. 1970 May;102(2):498–507. doi: 10.1128/jb.102.2.498-507.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson O. H., Holden J. T. Stimulation of arginine transport in osmotically shocked Escherichia coli W cells by purified arginine-binding protein fractions. J Biol Chem. 1969 May 25;244(10):2743–2749. [PubMed] [Google Scholar]