Abstract
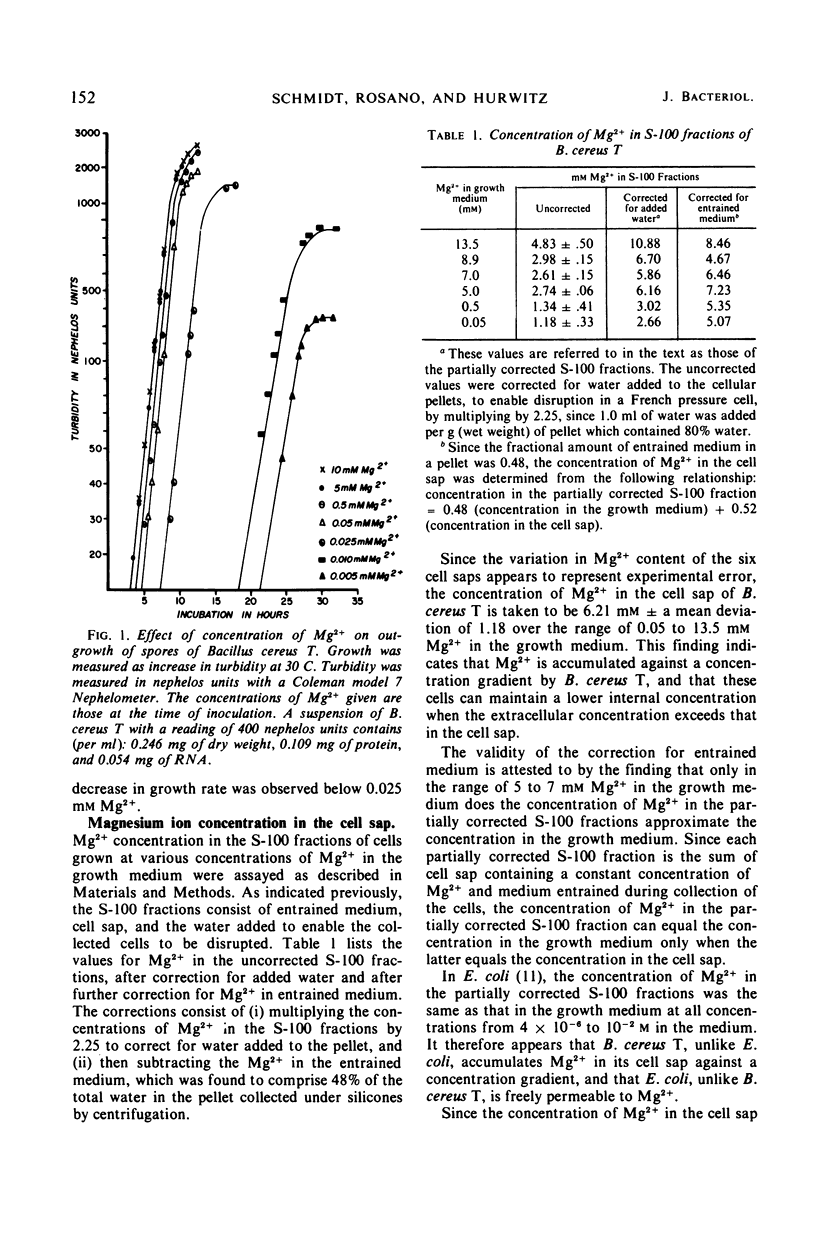
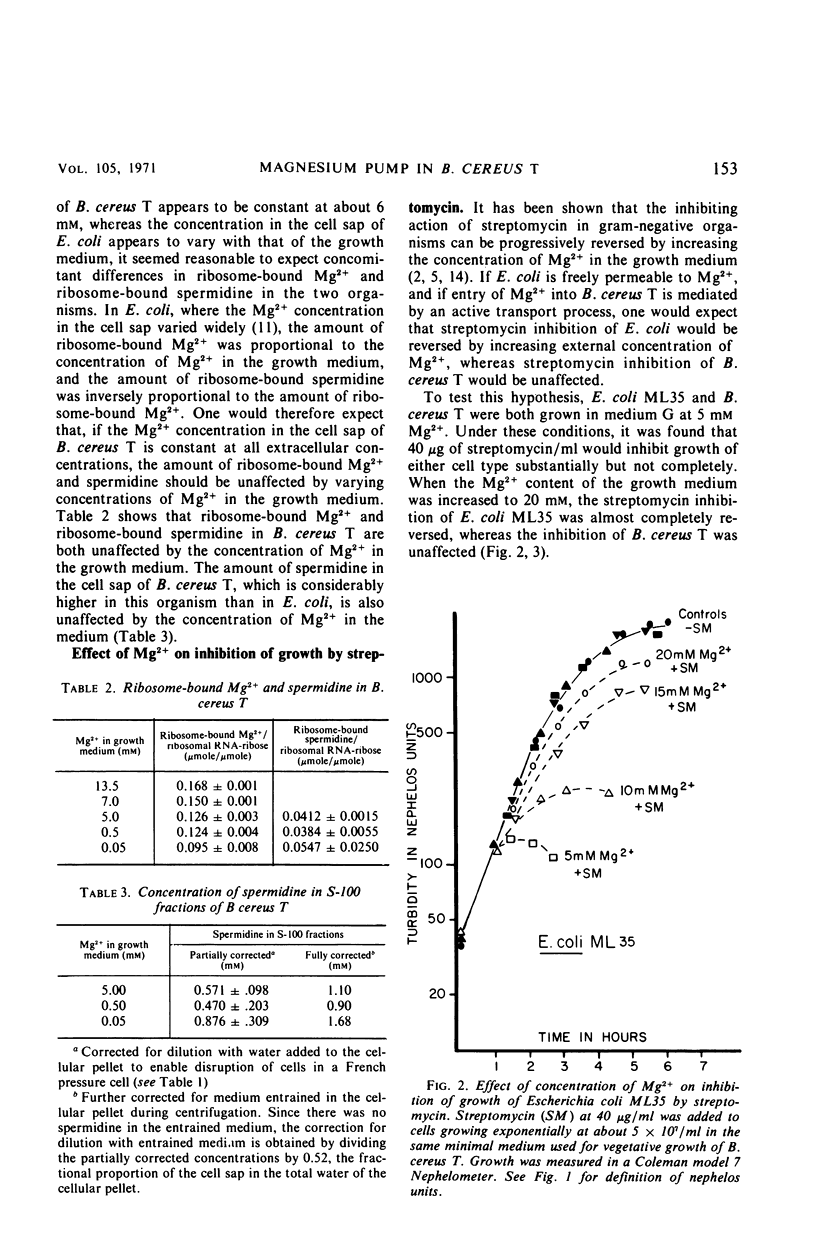
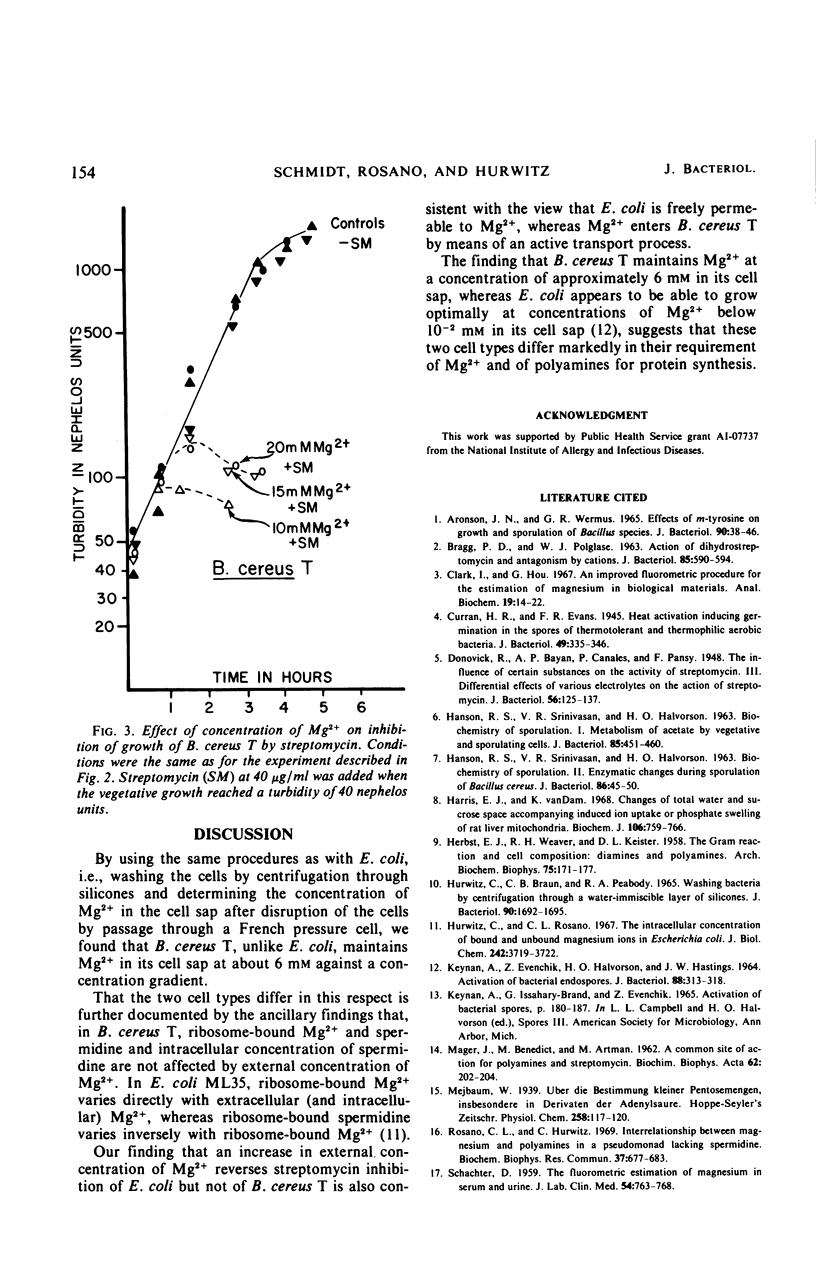
Unlike Escherichia coli, Bacillus cereus T appears to accumulate Mg2+ in its cell sap against a concentration gradient. Over a range of Mg2+ in the growth medium from 5 × 10−5 to 1.35 × 10−2m, the concentration of Mg2+ in the cell sap of B. cereus T was maintained at about 6 × 10−3m, and ribosome-bound Mg2+ and spermidine, as well as the spermidine concentration in the cell sap, appear to be unaffected by the concentration of Mg2+ in the growth medium. Inhibition of growth of E. coli by streptomycin is progressively reversed by increasing the concentration of Mg2+ in the growth medium above 5 mm. The finding that similar increases of Mg2+ in the growth medium did not reverse the inhibition of B. cereus T is also consistent with the conclusion that B. cereus T, unlike E. coli, accumulates Mg2+ to a constant concentration in its cell sap.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson J. N., Wermus G. R. Effects of m-Tyrosine on Growth and Sporulation of Bacillus Species. J Bacteriol. 1965 Jul;90(1):38–46. doi: 10.1128/jb.90.1.38-46.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAGG P. D., POLGLASE W. J. ACTION OF DIHYDROSTREPTOMYCIN AND ANTAGONISM BY CATIONS. J Bacteriol. 1963 Mar;85:590–594. doi: 10.1128/jb.85.3.590-594.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I., Hou G. An improved fluorometric procedure for the estimation of magnesium in biological materials. Anal Biochem. 1967 Apr;19(1):14–22. doi: 10.1016/0003-2697(67)90128-5. [DOI] [PubMed] [Google Scholar]
- Curran H. R., Evans F. R. Heat Activation Inducing Germination in the Spores of Thermotolerant and Thermophilic Aerobic Bacteria. J Bacteriol. 1945 Apr;49(4):335–346. doi: 10.1128/jb.49.4.335-346.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovick R., Bayan A. P., Canales P., Pansy F. The Influence of Certain Substances on the Activity of Streptomycin: III. Differential Effects of Various Electrolytes on the Action of Streptomycin. J Bacteriol. 1948 Jul;56(1):125–137. [PMC free article] [PubMed] [Google Scholar]
- HANSON R. S., SRINIVASAN V. R., HALVORSON H. O. BIOCHEMISTRY OF SPORULATION. II. ENZYMATIC CHANGES DURING SPORULATION OF BACILLUS CEREUS. J Bacteriol. 1963 Jul;86:45–50. doi: 10.1128/jb.86.1.45-50.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSON R. S., SRINIVASAN V. R., HALVORSON H. O. Biochemistry of sporulation. I. Metabolism of acetate by vegetative and sporulating cells. J Bacteriol. 1963 Feb;85:451–460. doi: 10.1128/jb.85.2.451-460.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERBST E. J., WEAVER R. H., KEISTER D. L. The gram reaction and cell composition: diamines and polyamines. Arch Biochem Biophys. 1958 May;75(1):171–177. doi: 10.1016/0003-9861(58)90407-7. [DOI] [PubMed] [Google Scholar]
- Harris E. J., van Dam K. Changes of total water and sucrose space accompanying induced ion uptake or phosphate swelling of rat liver mitochondria. Biochem J. 1968 Feb;106(3):759–766. doi: 10.1042/bj1060759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz C., Braun C. B., Peabody R. A. Washing bacteria by centrifugation through a water-immiscible layer of silicones. J Bacteriol. 1965 Dec;90(6):1692–1695. doi: 10.1128/jb.90.6.1692-1695.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz C., Rosano C. L. The intracellular concentration of bound and unbound magnesium ions in Escherichia coli. J Biol Chem. 1967 Aug 25;242(16):3719–3722. [PubMed] [Google Scholar]
- KEYNAN A., EVANCHIK Z., HALVORSON H. O., HASTINGS J. W. ACTIVATION OF BACTERIAL ENDOSPORES. J Bacteriol. 1964 Aug;88:313–318. doi: 10.1128/jb.88.2.313-318.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGER J., BENEDICT M., ARTMAN M. A common site of action of polyamines and streptomycin. Biochim Biophys Acta. 1962 Jul 30;62:202–204. doi: 10.1016/0006-3002(62)90519-x. [DOI] [PubMed] [Google Scholar]
- Rosano C. L., Hurwitz C. Interrelationship between magnesium and polyamines in a pseudomonad lacking spermidine. Biochem Biophys Res Commun. 1969 Nov 6;37(4):677–683. doi: 10.1016/0006-291x(69)90864-x. [DOI] [PubMed] [Google Scholar]
- SCHACHTER D. The fluorometric estimation of magnesium in serum and in urine. J Lab Clin Med. 1959 Nov;54:763–768. [PubMed] [Google Scholar]
- STEWART B. T., HALVORSON H. O. Studies on the spores of aerobic bacteria. I. The occurrence of alanine racemase. J Bacteriol. 1953 Feb;65(2):160–166. doi: 10.1128/jb.65.2.160-166.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb M. The utilization of magnesium by certain Gram-positive and Gram-negative bacteria. J Gen Microbiol. 1966 Jun;43(3):401–409. doi: 10.1099/00221287-43-3-401. [DOI] [PubMed] [Google Scholar]


