Abstract
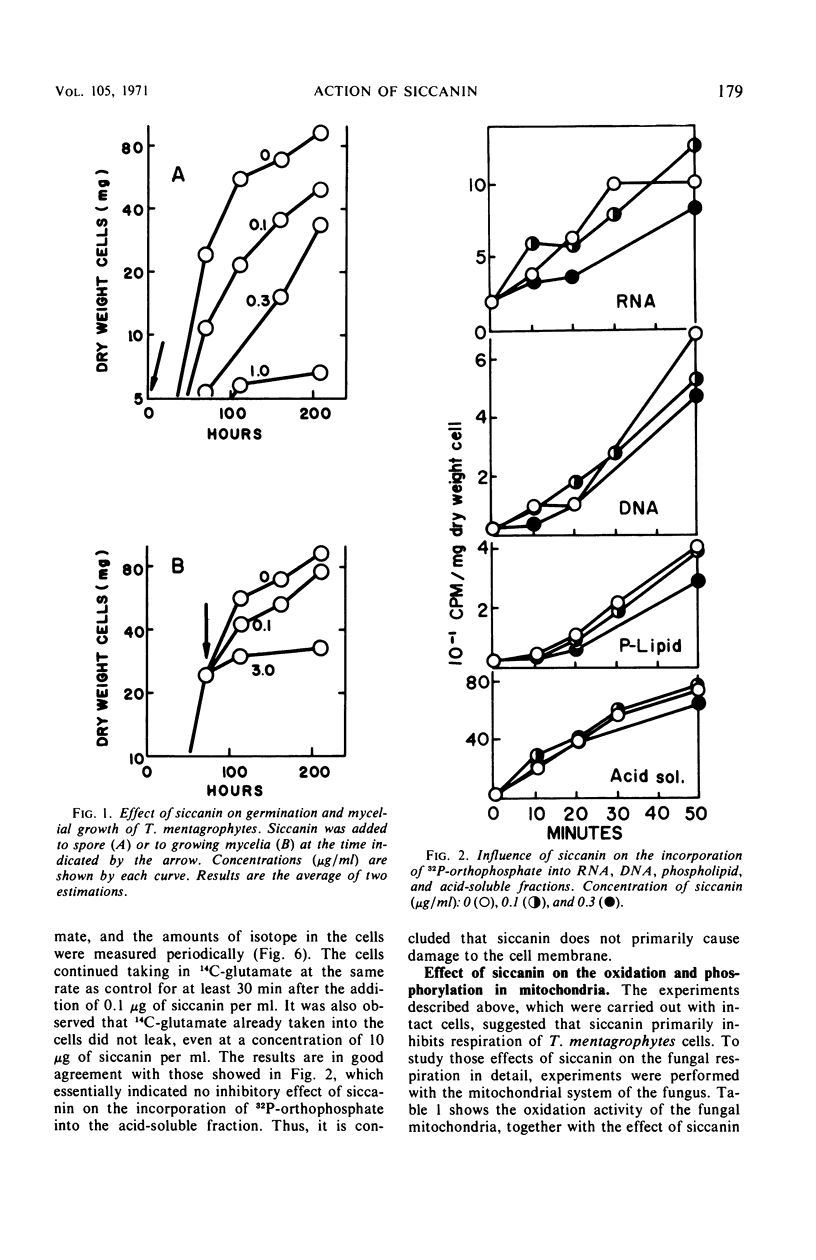
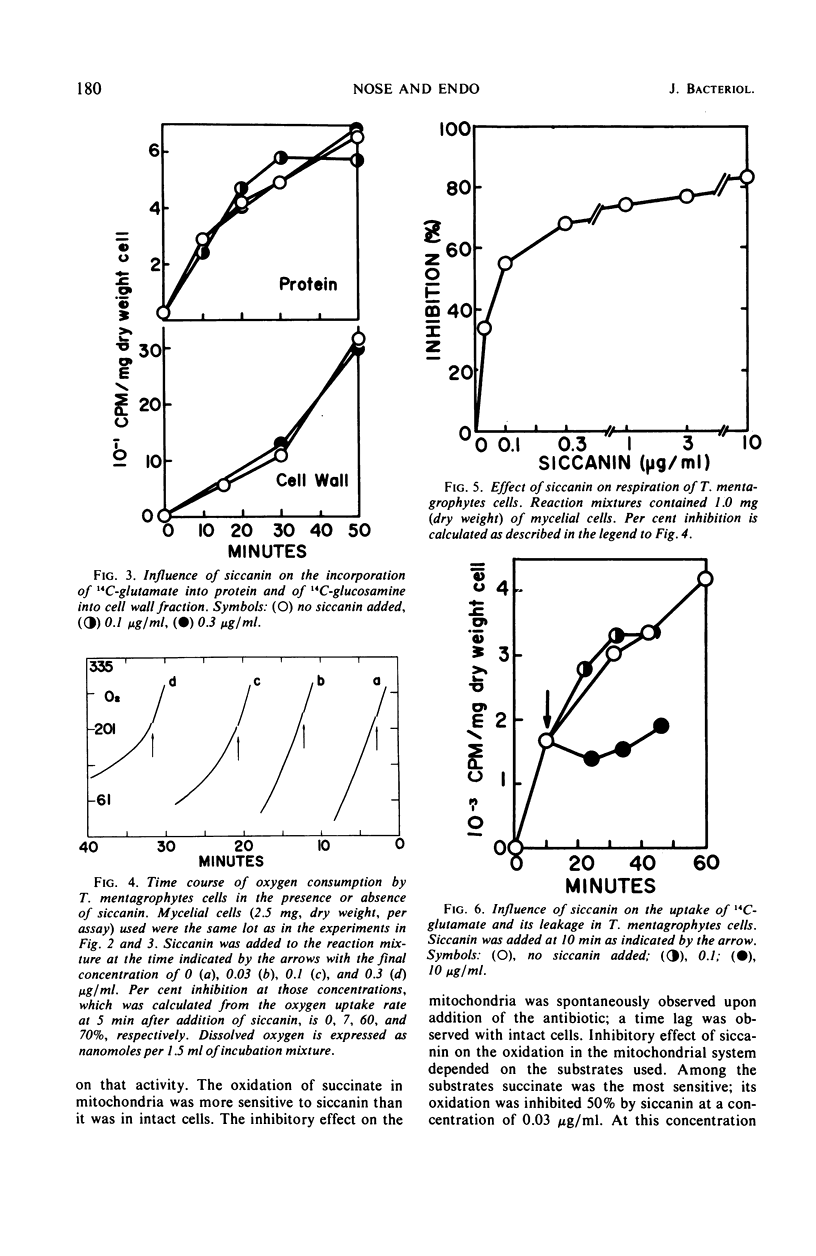
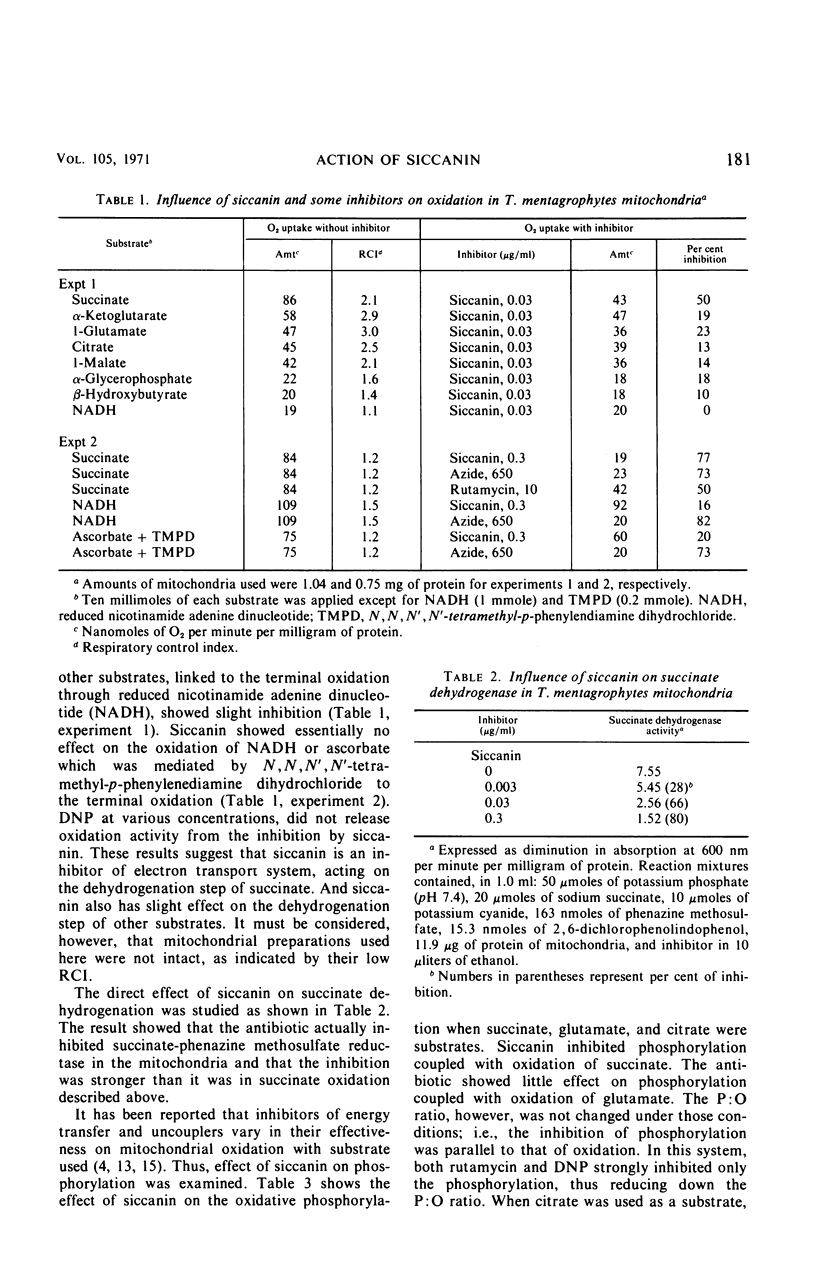
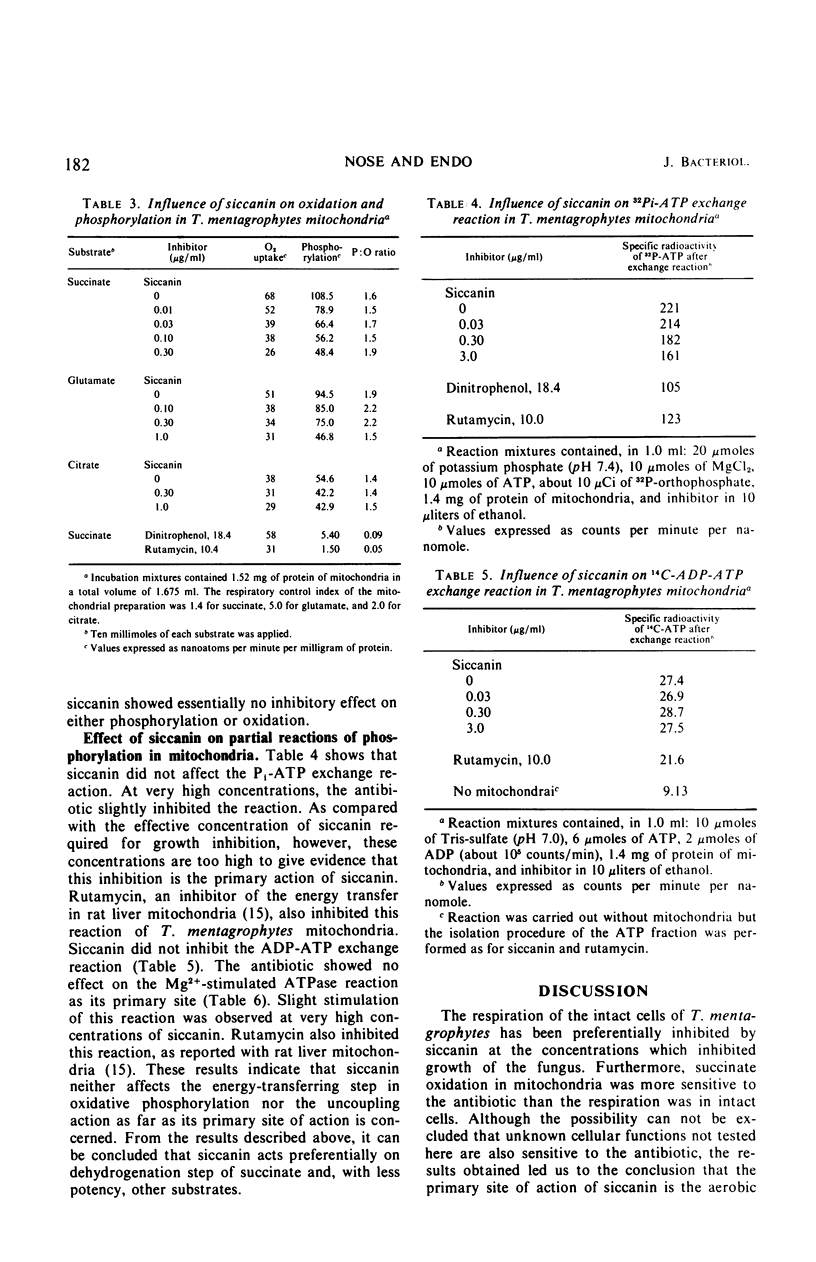
Siccanin at 3 μg/ml completely inhibited the growth of Trichophyton mentagrophytes. The primary site of action of siccanin on T. mentagrophytes is succinate dehydrogenase in the terminal electron transport system. At a concentration of siccanin giving 50% inhibition of growth (0.3 μg/ml), respiration of intact cells was inhibited more strongly than any other cellular functions tested, including the syntheses of cellular ribonucleic acid, deoxyribonucleic acid, phospholipid, protein, and cell wall fractions. In addition, at the same concentration siccanin did not cause any detectable damage in the permeability of the cells. Furthermore, the oxidation of succinate in mitochondrial preparation is more sensitive to the antibiotic than respiration in intact cells. Oxidation of other substrates tested was less sensitive to siccanin than that of succinate. The antibiotic inhibited both phosphorylation and oxidation, without causing changes in the P:O ratio. Siccanin at 0.03 μg/ml, which caused 50% inhibition of succinate oxidation in mitochondria, had effect neither on the exchange reaction between inorganic phosphate (Pi) and adenosine triphosphate (ATP) nor on that between adenosine diphosphate and ATP. An ATP phosphohydrolase activity was also insensitive to the antibiotic. At very high concentrations, however, the antibiotic slightly inhibited the Pi-ATP exchange reaction. From those results, it was concluded that siccanin inhibits fungal growth by inhibiting the respiratory electron transport system.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CONOVER T. E., PRAIRIE R. L., RACKER E. PARTIAL RESOLUTION OF THE ENZYMES CATALYZING OXIDATIVE PHOSPHORYLATION. III. A NEW COUPLING FACTOR REQUIRED BY SUBMITOCHONDRIAL PARTICLES EXTRACTED WITH PHOSPHATIDES. J Biol Chem. 1963 Aug;238:2831–2837. [PubMed] [Google Scholar]
- ELLS H. A. A colorimetric method for the assay of soluble succinic dehydrogenase and pyridinenucleotide-linked dehydrogenases. Arch Biochem Biophys. 1959 Dec;85:561–562. doi: 10.1016/0003-9861(59)90527-2. [DOI] [PubMed] [Google Scholar]
- HAGIHARA B. Techniques for the application of polarography to mitochondrial respiration. Biochim Biophys Acta. 1961 Jan 1;46:134–142. doi: 10.1016/0006-3002(61)90656-4. [DOI] [PubMed] [Google Scholar]
- Hirai K., Nozoe S., Tsuda K., Iitaka Y., Ishibashi K., Shirasaka M. The structure of siccainin. Tetrahedron Lett. 1967 Jun;23:2177–2179. doi: 10.1016/s0040-4039(00)90792-5. [DOI] [PubMed] [Google Scholar]
- JOHNSON R. B., FELDOTT G., LARDY H. A. The mode of action of the antibiotic, usnic acid. Arch Biochem. 1950 Oct;28(3):317–323. [PubMed] [Google Scholar]
- LARDY H. A., CONNELLY J. L., JOHNSON D. ANTIBIOTIC STUDIES. II. INHIBITION OF PHOSPHORYL TRANSFER IN MITOCHONDRIA BY OLIGOMYCIN AND AUROVERTIN. Biochemistry. 1964 Dec;3:1961–1968. doi: 10.1021/bi00900a030. [DOI] [PubMed] [Google Scholar]
- LARDY H. A., WITONSKY P., JOHNSON D. ANTIBIOTICS AS TOOLS FOR METABOLIC STUDIES. IV. COMPARATIVE EFFECTIVENESS OF OLIGOMYCINS A, B, C, AND RUTAMYCIN AS INHIBITORS OF PHOSPHORYL TRANSFER REACTIONS IN MITOCHONDRIA. Biochemistry. 1965 Mar;4:552–554. doi: 10.1021/bi00879a027. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- NEWTON B. A. The properties and mode of action of the polymyxins. Bacteriol Rev. 1956 Mar;20(1):14–27. doi: 10.1128/br.20.1.14-27.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIELSEN S. O., LEHNINGER A. L. Phosphorylation coupled to the oxidation of ferrocytochrome c. J Biol Chem. 1955 Aug;215(2):555–570. [PubMed] [Google Scholar]
- PULLMAN M. E., MONROY G. C. A NATURALLY OCCURRING INHIBITOR OF MITOCHONDRIAL ADENOSINE TRIPHOSPHATASE. J Biol Chem. 1963 Nov;238:3762–3769. [PubMed] [Google Scholar]
- WADKINS C. L., LEHNINGER A. L. The adenosine triphosphate-adenosine diphosphate exchange reaction of oxidative phosphorylation. J Biol Chem. 1958 Dec;233(6):1589–1597. [PubMed] [Google Scholar]
- WEISS B. AN ELECTRON MICROSCOPE AND BIOCHEMICAL STUDY OF NEUROSPORA CRASSA DURING DEVELOPMENT. J Gen Microbiol. 1965 Apr;39:85–94. doi: 10.1099/00221287-39-1-85. [DOI] [PubMed] [Google Scholar]


