Abstract
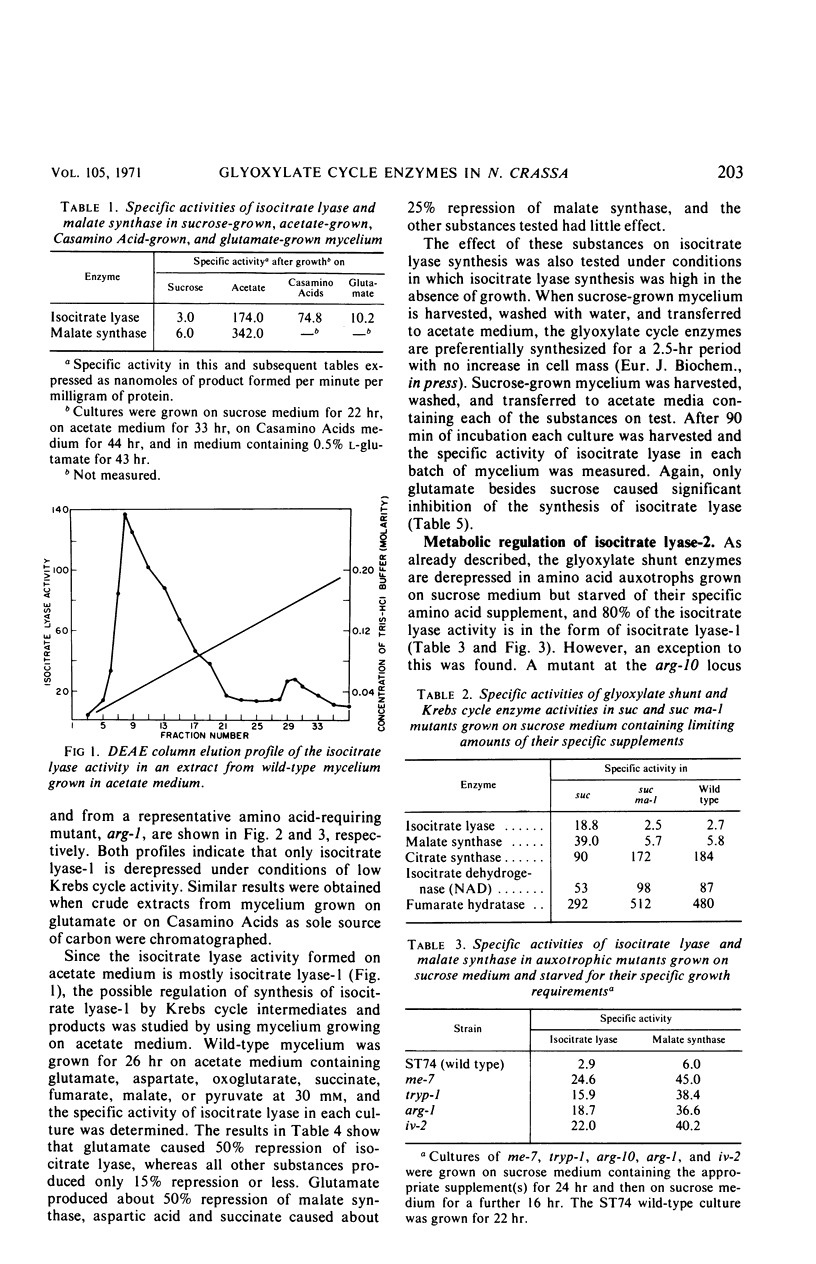
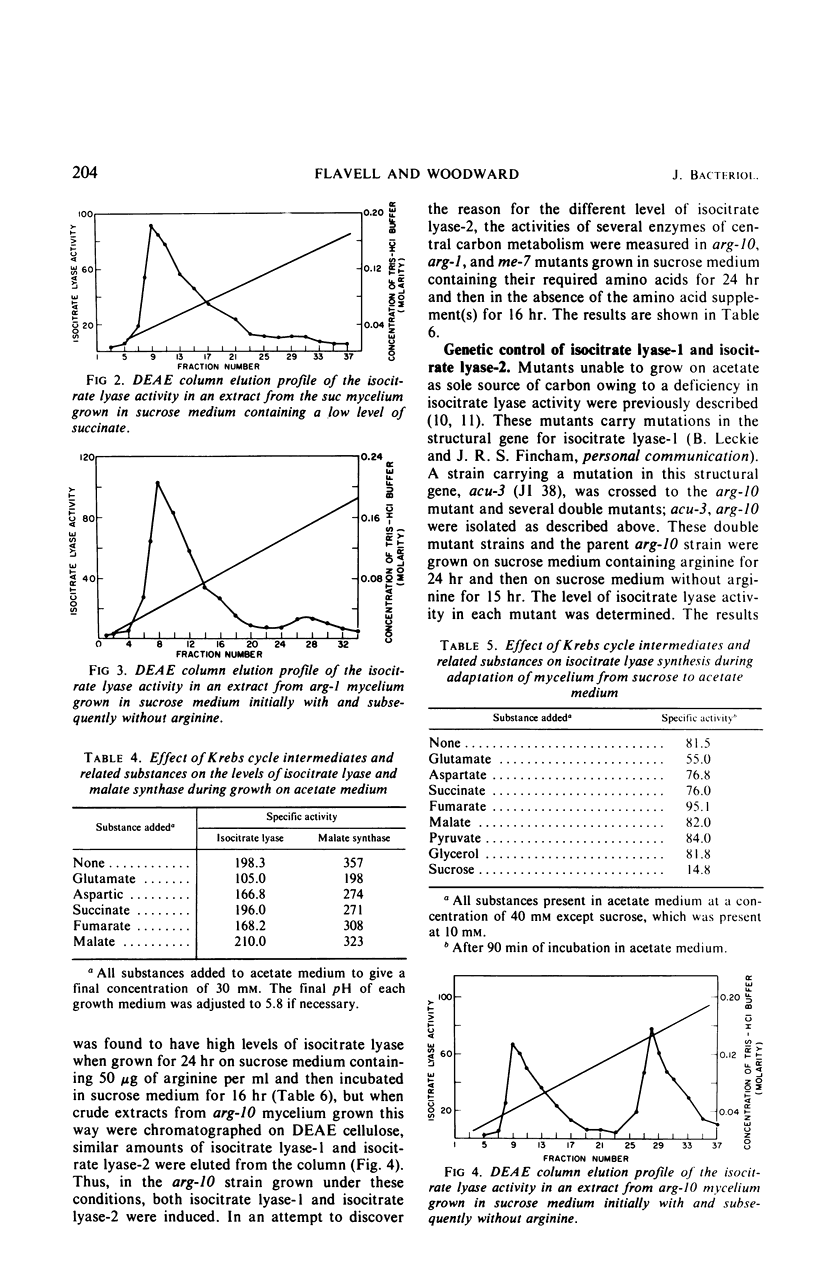
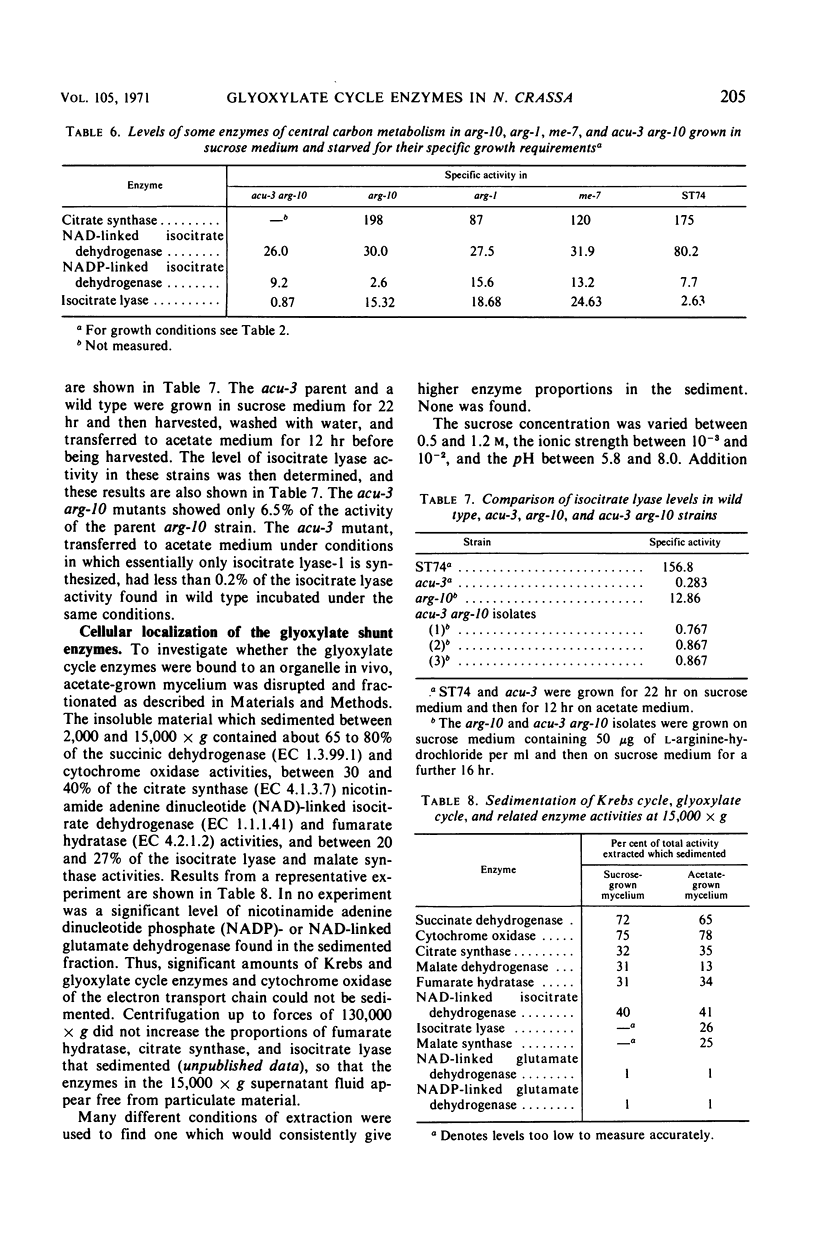
The glyoxylate shunt enzymes, isocitrate lyase and malate synthase, were present at high levels in mycelium grown on acetate as sole source of carbon, compared with mycelium grown on sucrose medium. The glyoxylate shunt activities were also elevated in mycelium grown on glutamate or Casamino Acids as sole source of carbon, and in amino acid-requiring auxotrophic mutants grown in sucrose medium containing limiting amounts of their required amino acid. Under conditions of enhanced catabolite repression in mutants grown in sucrose medium but starved of Krebs cycle intermediates, isocitrate lyase and malate synthase levels were derepressed compared with the levels in wild type grown on sucrose medium. This derepression did not occur in related mutants in which Krebs cycle intermediates were limiting growth but catabolite repression was not enhanced. No Krebs cycle intermediate tested produced an efficient repression of isocitrate lyase activity in acetate medium. Of the two forms of isocitrate lyase in Neurospora, isocitrate lyase-1 constituted over 80% of the isocitrate lyase activity in acetate-grown wild type and also in each of the cases already outlined in which the glyoxylate shunt activities were elevated on sucrose medium. On the basis of these results, it is concluded that the synthesis of isocitrate lyase-1 and malate synthase in Neurospora is regulated by a glycolytic intermediate or derivative. Our data suggest that isocitrate lyase-1 and isocitrate lyase-2 are the products of different structural genes. The metabolic roles of the two forms of isocitrate lyase and of the glyoxylate cycle are discussed on the basis of their metabolic control and intracellular localization.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armitt S., Roberts C. F., Kornberg H. L. The role of isocitrate lyase in Aspergillus Nidulans. FEBS Lett. 1970 Apr 16;7(3):231–234. doi: 10.1016/0014-5793(70)80168-5. [DOI] [PubMed] [Google Scholar]
- Ashworth J. M., Kornberg H. L. The anaplerotic fixation of carbon dioxide by Escherichia coli. Proc R Soc Lond B Biol Sci. 1966 Aug 16;165(999):179–188. doi: 10.1098/rspb.1966.0063. [DOI] [PubMed] [Google Scholar]
- Breidenbach R. W., Beevers H. Association of the glyoxylate cycle enzymes in a novel subcellular particle from castor bean endosperm. Biochem Biophys Res Commun. 1967 May 25;27(4):462–469. doi: 10.1016/s0006-291x(67)80007-x. [DOI] [PubMed] [Google Scholar]
- Brice C. B., Kornberg H. L. Genetic control of isocitrate lyase activity in Escherichia coli. J Bacteriol. 1968 Dec;96(6):2185–2186. doi: 10.1128/jb.96.6.2185-2186.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANVIN D. T., BEEVERS H. Sucrose synthesis from acetate in the germinating castor bean: kinetics and pathway. J Biol Chem. 1961 Apr;236:988–995. [PubMed] [Google Scholar]
- COLLINS J. F., KORNBERG H. L. The metabolism of C2 compounds in micro-organisms. 4. Synthesis of cell materials from acetate by Aspergillus niger. Biochem J. 1960 Dec;77:430–438. doi: 10.1042/bj0770430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter W. D., Beevers H. Distribution and Properties of Isocitritase in Plants. Plant Physiol. 1959 Jul;34(4):403–409. doi: 10.1104/pp.34.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- Flavell R. B., Fincham J. R. Acetate-nonutilizing mutants of Neurospora rassa. II. Biochemical deficiencies and the roles of certain enzymes. J Bacteriol. 1968 Mar;95(3):1063–1068. doi: 10.1128/jb.95.3.1063-1068.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell R. B., Fincham J. R. Acetate-onutilizing mutants of Neurospora crassa. I. Mutant isolation, complementation studies, and linkage relationships. J Bacteriol. 1968 Mar;95(3):1056–1062. doi: 10.1128/jb.95.3.1056-1062.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell R. B., Woodward D. O. The regulation of synthesis of Krebs cycle enzymes in Neurospora by catabolite and end product repression. Eur J Biochem. 1970 Apr;13(3):548–553. doi: 10.1111/j.1432-1033.1970.tb00959.x. [DOI] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- HOGG J. F., KORNBERG H. L. The metabolism of C2-compounds in micro-organisms. 9. Role of the glyoxylate cycle in protozoal glyconeogenesis. Biochem J. 1963 Mar;86:462–468. doi: 10.1042/bj0860462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrop L. C., Kornberg H. L. The role of isocitrate lyase in the metabolism of algae. Proc R Soc Lond B Biol Sci. 1966 Nov 15;166(1002):11–29. doi: 10.1098/rspb.1966.0082. [DOI] [PubMed] [Google Scholar]
- KORNBERG H. L., ELSDEN S. R. The metabolism of 2-carbon compounds by microorganisms. Adv Enzymol Relat Subj Biochem. 1961;23:401–470. doi: 10.1002/9780470122686.ch8. [DOI] [PubMed] [Google Scholar]
- KORNBERG H. L. Selective utilization of metabolic routes by Escherichia coli. Cold Spring Harb Symp Quant Biol. 1961;26:257–260. doi: 10.1101/sqb.1961.026.01.032. [DOI] [PubMed] [Google Scholar]
- KORNBERG H. L. THE ROLE OF ACETATE IN ISOCITRATE LYASE INDUCTION. Biochim Biophys Acta. 1963 Jul 9;73:517–519. doi: 10.1016/0006-3002(63)90456-6. [DOI] [PubMed] [Google Scholar]
- Kobr M. J., Bianchi D. E., Oulevey N., Turian G. The effect of oxygen tension on growth, conidiation, and alcohol production of Neurospora crassa. Can J Microbiol. 1967 Jul;13(7):805–809. doi: 10.1139/m67-106. [DOI] [PubMed] [Google Scholar]
- Kobr M. J., Turian G., Zimmerman E. J. Changes in enzymes regulating isocitrate breakdown in Neurospora crassa. Arch Mikrobiol. 1965 Oct 14;52(2):169–177. doi: 10.1007/BF00407726. [DOI] [PubMed] [Google Scholar]
- Kobr M. J., Vanderhaeghe F., Combépine G. Particulate enzymes of the glyoxylate cycle in Neurospora crassa. Biochem Biophys Res Commun. 1969 Nov 6;37(4):640–645. doi: 10.1016/0006-291x(69)90858-4. [DOI] [PubMed] [Google Scholar]
- Müller M., Hogg J. F., De Duve C. Distribution of tricarboxylic acid cycle enzymes and glyoxylate cycle enzymes between mitochondria and peroxisomes in Tetrahymena pyriformis. J Biol Chem. 1968 Oct 25;243(20):5385–5395. [PubMed] [Google Scholar]
- Polakis E. S., Bartley W. Changes in the intracellular concentrations of adenosine phosphates and nicotinamide nucleotides during the aerobic growth cycle of yeast on different carbon sources. Biochem J. 1966 Jun;99(3):521–533. doi: 10.1042/bj0990521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAUSS B. S. Oxalacetic carboxylase deficiency of the succinate-requiring mutants of Neurospora crassa. J Biol Chem. 1957 Mar;225(1):535–544. [PubMed] [Google Scholar]
- Sjogren R. E., Romano A. H. Evidence for multiple forms of isocitrate lyase in Neurospora crassa. J Bacteriol. 1967 May;93(5):1638–1643. doi: 10.1128/jb.93.5.1638-1643.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURIAN G., SEYDOUX J., VOLKMANN D. [Isocitratase activity and type of sporulation in Neurospora tetrasperma and N. sitophila, normal stain and microconidial mutant]. Pathol Microbiol (Basel) 1962;25:737–751. [PubMed] [Google Scholar]
- TURIAN G. [Glyoxylic acid cycle, alanine-glyoxylate transaminase and sexual differentiation in Allomyces and Neurospora]. Pathol Microbiol (Basel) 1961;24:819–839. [PubMed] [Google Scholar]


