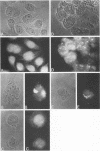
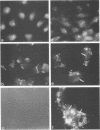
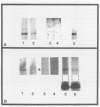
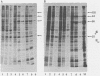
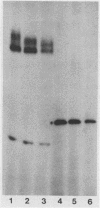
Abstract
It is clear from previous studies that host transcriptase or RNA polymerase II (pol II) has a role in poxvirus replication. To elucidate the participation of this enzyme further, in this study we examined several parameters related to pol II during the cycle of vaccinia virus infection in L-strain fibroblasts, HeLa cells, and L6H9 rat myoblasts. Nucleocytoplasmic transposition of pol II into virus factories and virions was assessed by immunofluorescence and immunoblotting by using anti-pol II immunoglobulin G. RNA polymerase activities were compared in nuclear extracts containing crude enzyme preparations. Rates of translation into cellular or viral polypeptides were ascertained by labeling with [35S]methionine. In L and HeLa cells, which produced vaccinia virus more abundantly, the rates of RNA polymerase and translation in controls and following infection were higher than in myoblasts. The data on synthesis and virus formation could be correlated with observations on transmigration of pol II, which was more efficient and complete in L and HeLa cells. The stimulus for pol II to leave the nucleus required the expression of both early and late viral functions. On the basis of current and past information, we suggest that mobilization of pol II depends on the efficiency of vaccina virus replication and furthermore that control over vaccinia virus production by the host is related to the content or availability (or both) of pol II in different cell types.
Full text
PDF

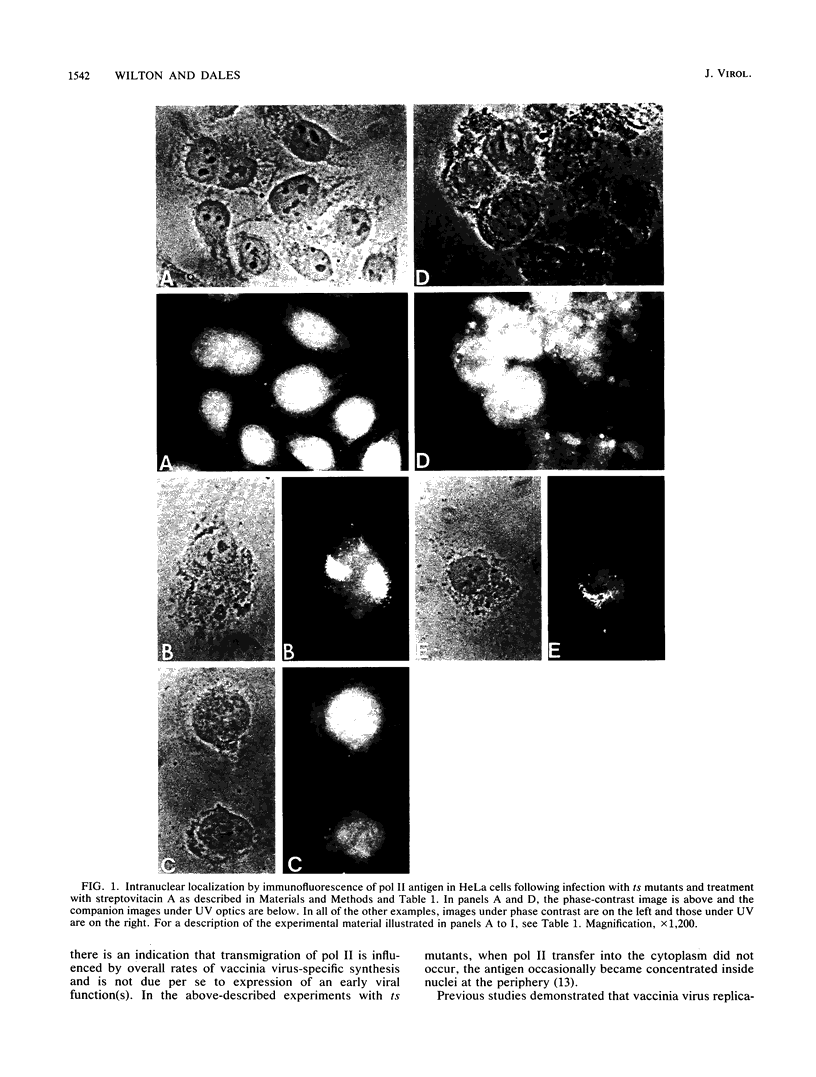
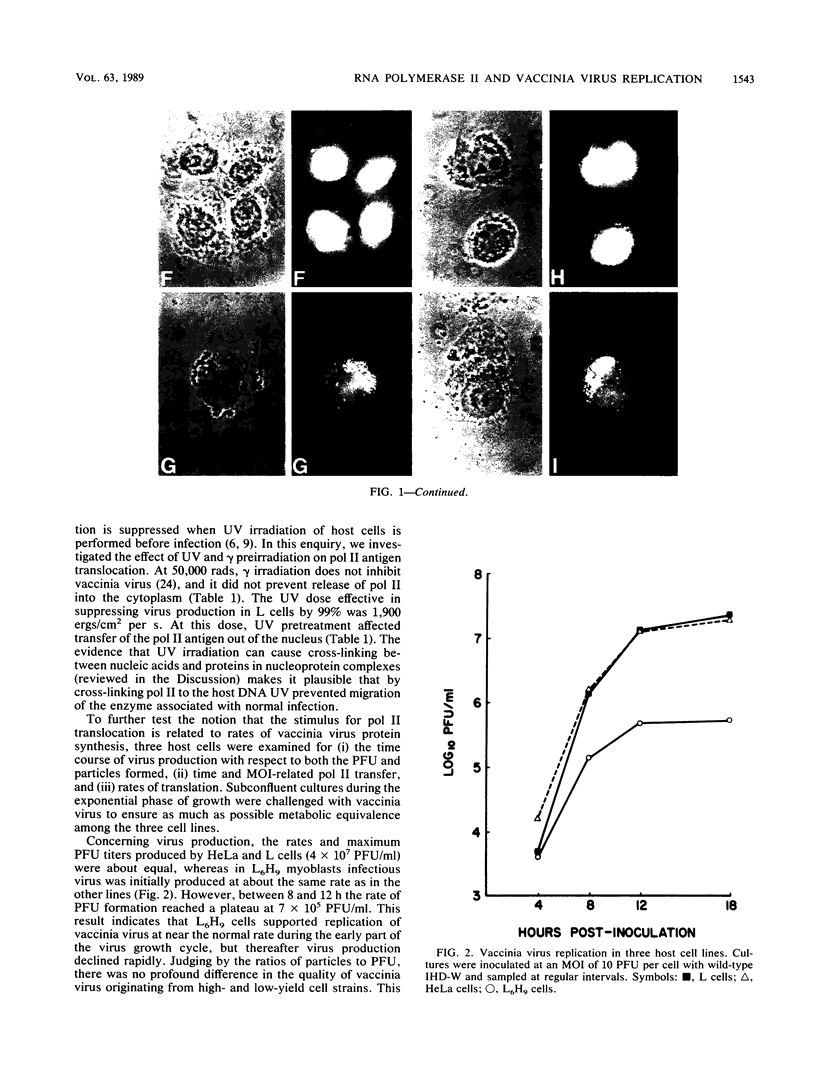
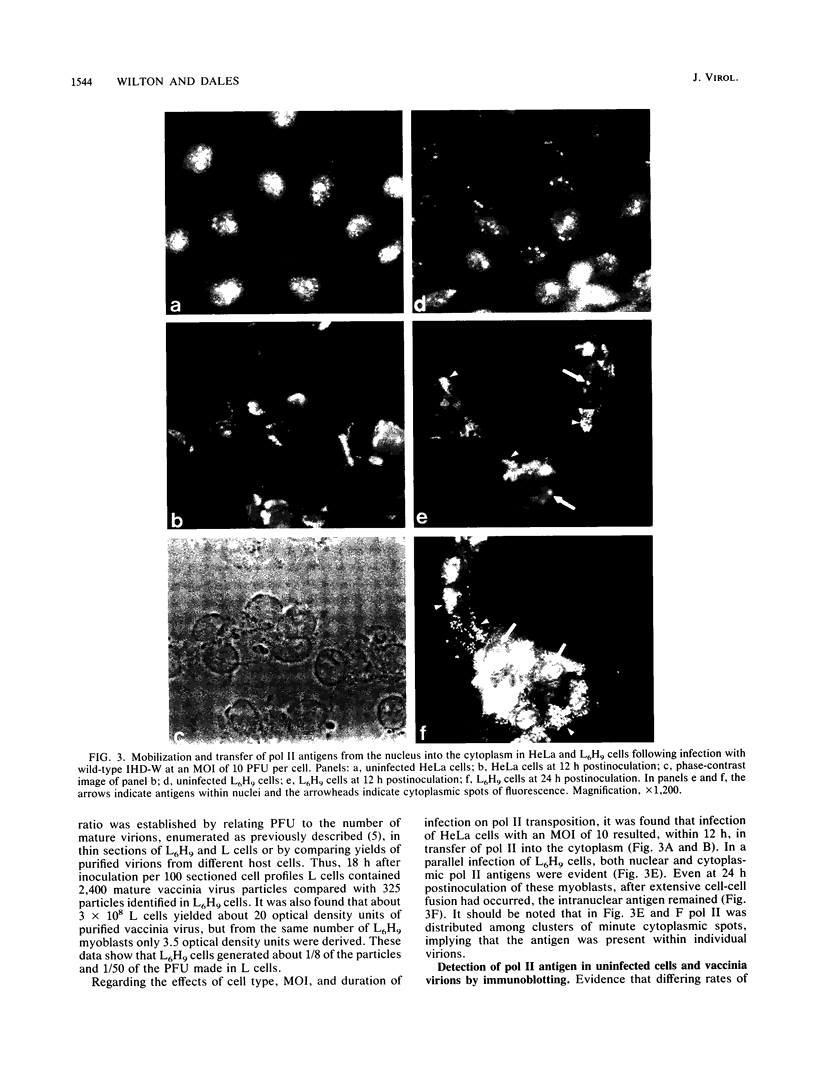



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colombo B., Felicetti L., Baglioni C. Inhibition of protein synthesis in reticulocytes by antibiotics. I. Effects on polysomes. Biochim Biophys Acta. 1966 Apr 18;119(1):109–119. doi: 10.1016/0005-2787(66)90043-8. [DOI] [PubMed] [Google Scholar]
- DALES S. The uptake and development of vaccinia virus in strain L cells followed with labeled viral deoxyribonucleic acid. J Cell Biol. 1963 Jul;18:51–72. doi: 10.1083/jcb.18.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S., Fujinami R. S., Oldstone M. B. Infection with vaccinia favors the selection of hybridomas synthesizing autoantibodies against intermediate filaments, one of them cross-reacting with the virus hemagglutinin. J Immunol. 1983 Sep;131(3):1546–1553. [PubMed] [Google Scholar]
- Dales S., Milovanovitch V., Pogo B. G., Weintraub S. B., Huima T., Wilton S., McFadden G. Biogenesis of vaccinia: isolation of conditional lethal mutants and electron microscopic characterization of their phenotypically expressed defects. Virology. 1978 Feb;84(2):403–428. doi: 10.1016/0042-6822(78)90258-1. [DOI] [PubMed] [Google Scholar]
- Dales S., Mosbach E. H. Vaccinia as a model for membrane biogenesis. Virology. 1968 Aug;35(4):564–583. doi: 10.1016/0042-6822(68)90286-9. [DOI] [PubMed] [Google Scholar]
- Hruby D. E., Lynn D. L., Kates J. R. Vaccinia virus replication requires active participation of the host cell transcriptional apparatus. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1887–1890. doi: 10.1073/pnas.76.4.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihashi Y., Matsumoto S., Dales S. Biogenesis of poxviruses: role of A-type inclusions and host cell membranes in virus dissemination. Virology. 1971 Dec;46(3):507–532. doi: 10.1016/0042-6822(71)90056-0. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. THE INTRACELLULAR UNCOATING OF POXVIRUS DNA. II. THE MOLECULAR BASIS OF THE UNCOATING PROCESS. J Mol Biol. 1964 Feb;8:277–288. doi: 10.1016/s0022-2836(64)80137-6. [DOI] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Poxvirus DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1967 Jul;58(1):134–141. doi: 10.1073/pnas.58.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Minnigan H., Moyer R. W. Intracellular location of rabbit poxvirus nucleic acid within infected cells as determined by in situ hybridization. J Virol. 1985 Sep;55(3):634–643. doi: 10.1128/jvi.55.3.634-643.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. K., Moyer R. W. Detection of a subunit of cellular Pol II within highly purified preparations of RNA polymerase isolated from rabbit poxvirus virions. Cell. 1986 Feb 28;44(4):587–596. doi: 10.1016/0092-8674(86)90268-0. [DOI] [PubMed] [Google Scholar]
- Moyer R. W. The role of the host cell nucleus in vaccinia virus morphogenesis. Virus Res. 1987 Sep;8(3):173–191. doi: 10.1016/0168-1702(87)90014-1. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Joklik W. K. Isolation and properties of the vaccinia virus DNA-dependent RNA polymerase. J Biol Chem. 1977 Oct 10;252(19):6930–6938. [PubMed] [Google Scholar]
- Pogo B. G., Dales S. Biogenesis of vaccinia: separation of early stages from maturation by means of hydroxyurea. Virology. 1971 Jan;43(1):144–151. doi: 10.1016/0042-6822(71)90232-7. [DOI] [PubMed] [Google Scholar]
- Robbins A., Dynan W. S., Greenleaf A., Tjian R. Affinity-purified antibody as a probe of RNA polymerase II subunit structure. J Mol Appl Genet. 1984;2(4):343–353. [PubMed] [Google Scholar]
- Roeder R. G., Rutter W. J. Specific nucleolar and nucleoplasmic RNA polymerases. Proc Natl Acad Sci U S A. 1970 Mar;65(3):675–682. doi: 10.1073/pnas.65.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHERER W. F., SYVERTON J. T., GEY G. O. Studies on the propagation in vitro of poliomyelitis viruses. IV. Viral multiplication in a stable strain of human malignant epithelial cells (strain HeLa) derived from an epidermoid carcinoma of the cervix. J Exp Med. 1953 May;97(5):695–710. doi: 10.1084/jem.97.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida H., Dales S. Biogenesis of vaccinia: carbohydrate of the hemagglutinin molecules. Virology. 1981 May;111(1):56–72. doi: 10.1016/0042-6822(81)90653-x. [DOI] [PubMed] [Google Scholar]
- Silver M., Dales S. Evidence against involvement of host transcription in the replication of vaccinia and herpes simplex viruses. Virology. 1982 Apr 15;118(1):214–218. doi: 10.1016/0042-6822(82)90334-8. [DOI] [PubMed] [Google Scholar]
- Silver M., Dales S. Potential of intense gamma-irradiation of host cells for analysis of virus-specified transcription and replication. Can J Biochem. 1982 Mar;60(3):279–283. doi: 10.1139/o82-033. [DOI] [PubMed] [Google Scholar]
- Silver M., McFadden G., Wilton S., Dales S. Biogenesis of poxviruses: role for the DNA-dependent RNA polymerase II of the host during expression of late functions. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4122–4125. doi: 10.1073/pnas.76.8.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers D. G., Pearson M. L., Ingles C. J. Isolation and characterization of an alpha-amanitin-resistant rat myoblast mutant cell line possessing alpha-amanitin-resistant RNA polymerase II. J Biol Chem. 1975 Jul 10;250(13):4825–4831. [PubMed] [Google Scholar]
- Stern W., Dales S. Biogenesis of vaccinia: isolation and characterization of a surface component that elicits antibody suppressing infectivity and cell-cell fusion. Virology. 1976 Nov;75(1):232–241. doi: 10.1016/0042-6822(76)90022-2. [DOI] [PubMed] [Google Scholar]
- Strniste G. F., Smith D. A. Induction of stable linkage between the deoxyribonucleic acid dependent ribonucleic acid polymerase and d(A-T)n-d(A-T)n by ultraviolet light. Biochemistry. 1974 Jan 29;13(3):485–493. doi: 10.1021/bi00700a014. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenmakers A. J., Reinders R. J., van Venrooij W. J. Cross-linking of mRNA to proteins by irradiation of intact cells with ultraviolet light. Eur J Biochem. 1980 Nov;112(2):323–330. doi: 10.1111/j.1432-1033.1980.tb07207.x. [DOI] [PubMed] [Google Scholar]
- Weeks J. R., Coulter D. E., Greenleaf A. L. Immunological studies of RNA polymerase II using antibodies to subunits of Drosophila and wheat germ enzyme. J Biol Chem. 1982 May 25;257(10):5884–5892. [PubMed] [Google Scholar]
- Wilton S., Dales S. Influence of RNA polymerase II upon vaccinia virus-related translation examined by means of alpha-amanitin. Virus Res. 1986 Sep;5(4):323–341. doi: 10.1016/0168-1702(86)90027-4. [DOI] [PubMed] [Google Scholar]
- Wilton S., Gordon J., Dales S. Identification of antigenic determinants by polyclonal and hybridoma antibodies induced during the course of infection by vaccinia virus. Virology. 1986 Jan 15;148(1):84–96. doi: 10.1016/0042-6822(86)90405-8. [DOI] [PubMed] [Google Scholar]
- Zylber E. A., Penman S. Products of RNA polymerases in HeLa cell nuclei. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2861–2865. doi: 10.1073/pnas.68.11.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eekelen C. A., van Venrooij W. J. hnRNA and its attachment to a nuclear protein matrix. J Cell Biol. 1981 Mar;88(3):554–563. doi: 10.1083/jcb.88.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]