Abstract
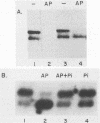
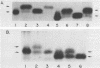
The shift in mobility on sodium dodecyl sulfate-polyacrylamide gel electrophoresis that is characteristic of the adenovirus E1A proteins is the result of posttranslational modification. In the present study, we demonstrate that phosphorylation of bacterially produced E1A in higher cell extracts occurs on serine and is responsible for the mobility shift. E1A protein expressed in Saccharomyces cerevisiae also undergoes the mobility shift due to serine phosphorylation. Site-directed mutagenesis was used to identify the serine residue responsible for the mobility shift. Six serine residues were altered to glycine within E1A. Substitution at serine residue 89 was shown to selectively prevent the mobility shift of both the 289R and 243R E1A proteins. We conclude that phosphorylation at serine 89 is the specific modification responsible for the mobility shift of E1A. Moreover, we demonstrate that the Ser-89-to-Gly mutation has no effect on trans activation or complementation of an E1A-deficient adenovirus. In contrast, the mutant protein does significantly reduce both the repression and transformation efficiency of E1A. The five other Ser-to-Gly mutation were also examined for functional effects. None affected trans activation, whereas repression and transformation functions were affected. One mutant affected transformation without affecting repression, suggesting that these functions are to some degree also separable. The relevance of phosphorylation to structure and activity of E1A and other nuclear oncogene proteins is discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Ramsay G., Bishop J. M., Pfeifer S. O., Colby W. W., Levinson A. D. Identification of nuclear proteins encoded by viral and cellular myc oncogenes. Nature. 1983 Nov 17;306(5940):274–277. doi: 10.1038/306274a0. [DOI] [PubMed] [Google Scholar]
- Barber J. R., Verma I. M. Modification of fos proteins: phosphorylation of c-fos, but not v-fos, is stimulated by 12-tetradecanoyl-phorbol-13-acetate and serum. Mol Cell Biol. 1987 Jun;7(6):2201–2211. doi: 10.1128/mcb.7.6.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann E. A. DNA-binding properties of phosphorylated and dephosphorylated D2-T antigen, a simian-virus-40 T-antigen-related protein. Eur J Biochem. 1985 Mar 15;147(3):495–501. doi: 10.1111/j.0014-2956.1985.00495.x. [DOI] [PubMed] [Google Scholar]
- Berk A. J. Adenovirus promoters and E1A transactivation. Annu Rev Genet. 1986;20:45–79. doi: 10.1146/annurev.ge.20.120186.000401. [DOI] [PubMed] [Google Scholar]
- Bockus B. J., Schaffhausen B. Phosphorylation of polyomavirus large T antigen: effects of viral mutations and cell growth state. J Virol. 1987 Apr;61(4):1147–1154. doi: 10.1128/jvi.61.4.1147-1154.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli E., Hen R., Chambon P. Adenovirus-2 E1A products repress enhancer-induced stimulation of transcription. Nature. 1984 Dec 13;312(5995):608–612. doi: 10.1038/312608a0. [DOI] [PubMed] [Google Scholar]
- Chlebowski J. F., Mabrey S., Falk M. C. Calorimetry of alkaline phosphatase. Stability of the monomer and effect of metal ion and phosphate binding on dimer stability. J Biol Chem. 1979 Jul 10;254(13):5745–5753. [PubMed] [Google Scholar]
- Curran T., Miller A. D., Zokas L., Verma I. M. Viral and cellular fos proteins: a comparative analysis. Cell. 1984 Feb;36(2):259–268. doi: 10.1016/0092-8674(84)90219-8. [DOI] [PubMed] [Google Scholar]
- Ferguson B., Jones N., Richter J., Rosenberg M. Adenovirus E1a gene product expressed at high levels in Escherichia coli is functional. Science. 1984 Jun 22;224(4655):1343–1346. doi: 10.1126/science.6374895. [DOI] [PubMed] [Google Scholar]
- Ferguson B., Krippl B., Andrisani O., Jones N., Westphal H., Rosenberg M. E1A 13S and 12S mRNA products made in Escherichia coli both function as nucleus-localized transcription activators but do not directly bind DNA. Mol Cell Biol. 1985 Oct;5(10):2653–2661. doi: 10.1128/mcb.5.10.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson B., Pritchard M. L., Feild J., Rieman D., Greig R. G., Poste G., Rosenberg M. Isolation and analysis of an Abelson murine leukemia virus-encoded tyrosine-specific kinase produced in Escherichia coli. J Biol Chem. 1985 Mar 25;260(6):3652–3657. [PubMed] [Google Scholar]
- Ferguson B., Rosenberg M., Krippl B. Transfer of functional adenovirus E1A transcription activator proteins into mammalian cells by protoplast fusion. J Biol Chem. 1986 Nov 5;261(31):14760–14763. [PubMed] [Google Scholar]
- Franza B. R., Jr, Maruyama K., Garrels J. I., Ruley H. E. In vitro establishment is not a sufficient prerequisite for transformation by activated ras oncogenes. Cell. 1986 Feb 14;44(3):409–418. doi: 10.1016/0092-8674(86)90462-9. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman J. A., Clark P. E., Lee M. C., Debouck C., Rosenberg M. Regulation of the yeast metallothionein gene. Gene. 1986;48(1):13–22. doi: 10.1016/0378-1119(86)90347-1. [DOI] [PubMed] [Google Scholar]
- Grässer F. A., Mann K., Walter G. Removal of serine phosphates from simian virus 40 large T antigen increases its ability to stimulate DNA replication in vitro but has no effect on ATPase and DNA binding. J Virol. 1987 Nov;61(11):3373–3380. doi: 10.1128/jvi.61.11.3373-3380.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann S. R., Abrams H. D., Rohrschneider L. R., Eisenman R. N. Proteins encoded by v-myc and c-myc oncogenes: identification and localization in acute leukemia virus transformants and bursal lymphoma cell lines. Cell. 1983 Oct;34(3):789–798. doi: 10.1016/0092-8674(83)90535-4. [DOI] [PubMed] [Google Scholar]
- Harlow E., Franza B. R., Jr, Schley C. Monoclonal antibodies specific for adenovirus early region 1A proteins: extensive heterogeneity in early region 1A products. J Virol. 1985 Sep;55(3):533–546. doi: 10.1128/jvi.55.3.533-546.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassauer M., Scheidtmann K. H., Walter G. Mapping of phosphorylation sites in polyomavirus large T antigen. J Virol. 1986 Jun;58(3):805–816. doi: 10.1128/jvi.58.3.805-816.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hen R., Borrelli E., Fromental C., Sassone-Corsi P., Chambon P. A mutated polyoma virus enhancer which is active in undifferentiated embryonal carcinoma cells is not repressed by adenovirus-2 E1A products. Nature. 1986 May 15;321(6067):249–251. doi: 10.1038/321249a0. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Richter J. D., Weeks D. L., Smith L. D. Regulation of adenovirus transcription by an E1a gene in microinjected Xenopus laevis oocytes. Mol Cell Biol. 1983 Dec;3(12):2131–2142. doi: 10.1128/mcb.3.12.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D., Smith A. E. In vitro mutagenesis of a putative DNA binding domain of SV40 large-T. Virology. 1984 Nov;139(1):109–137. doi: 10.1016/0042-6822(84)90334-9. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Miller J. S., Porter D., Roberts B. E. E1a regions of the human adenoviruses and of the highly oncogenic simian adenovirus 7 are closely related. J Virol. 1985 Feb;53(2):399–409. doi: 10.1128/jvi.53.2.399-409.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krippl B., Andrisani O., Jones N., Westphal H., Rosenberg M., Ferguson B. Adenovirus type 12 E1A protein expressed in Escherichia coli is functional upon transfer by microinjection or protoplast fusion into mammalian cells. J Virol. 1986 Aug;59(2):420–427. doi: 10.1128/jvi.59.2.420-427.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krippl B., Ferguson B., Rosenberg M., Westphal H. Functions of purified E1A protein microinjected into mammalian cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6988–6992. doi: 10.1073/pnas.81.22.6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff T., Elkaim R., Goding C. R., Jalinot P., Sassone-Corsi P., Perricaudet M., Kédinger C., Chambon P. Individual products of the adenovirus 12S and 13S EIa mRNAs stimulate viral EIIa and EIII expression at the transcriptional level. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4381–4385. doi: 10.1073/pnas.81.14.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie J. W., Green M., Green M. R. An adenovirus E1a protein region required for transformation and transcriptional repression. Cell. 1986 Sep 26;46(7):1043–1051. doi: 10.1016/0092-8674(86)90704-x. [DOI] [PubMed] [Google Scholar]
- Lillie J. W., Loewenstein P. M., Green M. R., Green M. Functional domains of adenovirus type 5 E1a proteins. Cell. 1987 Sep 25;50(7):1091–1100. doi: 10.1016/0092-8674(87)90175-9. [DOI] [PubMed] [Google Scholar]
- Lucher L. A., Loewenstein P. M., Green M. Phosphorylation in vitro of Escherichia coli-produced 235R and 266R tumor antigens encoded by human adenovirus type 12 early transformation region 1A. J Virol. 1985 Oct;56(1):183–193. doi: 10.1128/jvi.56.1.183-193.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacConnell W. P., Verma I. M. Expression of FBJ-MSV oncogene (fos) product in bacteria. Virology. 1983 Dec;131(2):367–374. doi: 10.1016/0042-6822(83)90504-4. [DOI] [PubMed] [Google Scholar]
- Matlashewski G., Lamb P., Pim D., Peacock J., Crawford L., Benchimol S. Isolation and characterization of a human p53 cDNA clone: expression of the human p53 gene. EMBO J. 1984 Dec 20;3(13):3257–3262. doi: 10.1002/j.1460-2075.1984.tb02287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto C., Smith G. E., Farrell-Towt J., Chizzonite R., Summers M. D., Ju G. Production of human c-myc protein in insect cells infected with a baculovirus expression vector. Mol Cell Biol. 1985 Oct;5(10):2860–2865. doi: 10.1128/mcb.5.10.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran E., Grodzicker T., Roberts R. J., Mathews M. B., Zerler B. Lytic and transforming functions of individual products of the adenovirus E1A gene. J Virol. 1986 Mar;57(3):765–775. doi: 10.1128/jvi.57.3.765-775.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran E., Mathews M. B. Multiple functional domains in the adenovirus E1A gene. Cell. 1987 Jan 30;48(2):177–178. doi: 10.1016/0092-8674(87)90418-1. [DOI] [PubMed] [Google Scholar]
- Mott J. E., Grant R. A., Ho Y. S., Platt T. Maximizing gene expression from plasmid vectors containing the lambda PL promoter: strategies for overproducing transcription termination factor rho. Proc Natl Acad Sci U S A. 1985 Jan;82(1):88–92. doi: 10.1073/pnas.82.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. Mechanism of activation of early viral transcription by the adenovirus E1A gene product. Cell. 1981 Oct;26(2 Pt 2):213–220. doi: 10.1016/0092-8674(81)90304-4. [DOI] [PubMed] [Google Scholar]
- Ramsay G., Hayman M. J., Bister K. Phosphorylation of specific sites in the gag-myc polyproteins encoded by MC29-type viruses correlates with their transforming ability. EMBO J. 1982;1(9):1111–1116. doi: 10.1002/j.1460-2075.1982.tb01305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Richter J. D., Slavicek J. M., Schneider J. F., Jones N. C. Heterogeneity of adenovirus type 5 E1A proteins: multiple serine phosphorylations induce slow-migrating electrophoretic variants but do not affect E1A-induced transcriptional activation or transformation. J Virol. 1988 Jun;62(6):1948–1955. doi: 10.1128/jvi.62.6.1948-1955.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J. D., Young P., Jones N. C., Krippl B., Rosenberg M., Ferguson B. A first exon-encoded domain of E1A sufficient for posttranslational modification, nuclear-localization, and induction of adenovirus E3 promoter expression in Xenopus oocytes. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8434–8438. doi: 10.1073/pnas.82.24.8434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe D. T., Yee S. P., Otis J., Graham F. L., Branton P. E. Characterization of human adenovirus type 5 early region 1A polypeptides using antitumor sera and an antiserum specific for the carboxy terminus. Virology. 1983 Jun;127(2):253–271. doi: 10.1016/0042-6822(83)90142-3. [DOI] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Samad A., Anderson C. W., Carroll R. B. Mapping of phosphomonoester and apparent phosphodiester bonds of the oncogene product p53 from simian virus 40-transformed 3T3 cells. Proc Natl Acad Sci U S A. 1986 Feb;83(4):897–901. doi: 10.1073/pnas.83.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H. Phosphorylation of simian virus 40 large T antigen: cytoplasmic and nuclear phophorylation sites differ in their metabolic stability. Virology. 1986 Apr 15;150(1):85–95. [PubMed] [Google Scholar]
- Schneider J. F., Fisher F., Goding C. R., Jones N. C. Mutational analysis of the adenovirus E1a gene: the role of transcriptional regulation in transformation. EMBO J. 1987 Jul;6(7):2053–2060. doi: 10.1002/j.1460-2075.1987.tb02470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif I., Khoury G., Dhar R. The genome of human papovavirus BKV. Cell. 1979 Dec;18(4):963–977. doi: 10.1016/0092-8674(79)90209-5. [DOI] [PubMed] [Google Scholar]
- Simmons D. T., Chou W., Rodgers K. Phosphorylation downregulates the DNA-binding activity of simian virus 40 T antigen. J Virol. 1986 Dec;60(3):888–894. doi: 10.1128/jvi.60.3.888-894.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler R., Bruner M., Dalie B., Harter M. L. Activation of adenovirus promoters by the adenovirus E1A protein in cell-free extracts. Science. 1987 Aug 28;237(4818):1044–1046. doi: 10.1126/science.2956686. [DOI] [PubMed] [Google Scholar]
- Tremblay M. L., McGlade C. J., Gerber G. E., Branton P. E. Identification of the phosphorylation sites in early region 1A proteins of adenovirus type 5 by amino acid sequencing of peptide fragments. J Biol Chem. 1988 May 5;263(13):6375–6383. [PubMed] [Google Scholar]
- Tsukamoto A. S., Ponticelli A., Berk A. J., Gaynor R. B. Genetic mapping of a major site of phosphorylation in adenovirus type 2 E1A proteins. J Virol. 1986 Jul;59(1):14–22. doi: 10.1128/jvi.59.1.14-22.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Curran T., Müller R., Verma I. M. Analysis of FBJ-MuSV provirus and c-fos (mouse) gene reveals that viral and cellular fos gene products have different carboxy termini. Cell. 1983 Apr;32(4):1241–1255. doi: 10.1016/0092-8674(83)90306-9. [DOI] [PubMed] [Google Scholar]
- Van Roy F., Fransen L., Fiers W. Phosphorylation patterns of tumour antigens in cells lytically infected or transformed by simian virus 40. J Virol. 1981 Oct;40(1):28–44. doi: 10.1128/jvi.40.1.28-44.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velcich A., Ziff E. Adenovirus E1a proteins repress transcription from the SV40 early promoter. Cell. 1985 Mar;40(3):705–716. doi: 10.1016/0092-8674(85)90219-3. [DOI] [PubMed] [Google Scholar]
- Velcich A., Ziff E. Adenovirus E1a ras cooperation activity is separate from its positive and negative transcription regulatory functions. Mol Cell Biol. 1988 May;8(5):2177–2183. doi: 10.1128/mcb.8.5.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt R. A., Shatzman A. R., Rosenberg M. Expression and characterization of the human c-myc DNA-binding protein. Mol Cell Biol. 1985 Mar;5(3):448–456. doi: 10.1128/mcb.5.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A. The action of oncogenes in the cytoplasm and nucleus. Science. 1985 Nov 15;230(4727):770–776. doi: 10.1126/science.2997917. [DOI] [PubMed] [Google Scholar]
- Whyte P., Ruley H. E., Harlow E. Two regions of the adenovirus early region 1A proteins are required for transformation. J Virol. 1988 Jan;62(1):257–265. doi: 10.1128/jvi.62.1.257-265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee S. P., Branton P. E. Analysis of multiple forms of human adenovirus type 5 E1A polypeptides using an anti-peptide antiserum specific for the amino terminus. Virology. 1985 Oct 30;146(2):315–322. doi: 10.1016/0042-6822(85)90015-7. [DOI] [PubMed] [Google Scholar]
- Yee S. P., Rowe D. T., Tremblay M. L., McDermott M., Branton P. E. Identification of human adenovirus early region 1 products by using antisera against synthetic peptides corresponding to the predicted carboxy termini. J Virol. 1983 Jun;46(3):1003–1013. doi: 10.1128/jvi.46.3.1003-1013.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerler B., Roberts R. J., Mathews M. B., Moran E. Different functional domains of the adenovirus E1A gene are involved in regulation of host cell cycle products. Mol Cell Biol. 1987 Feb;7(2):821–829. doi: 10.1128/mcb.7.2.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ormondt H., Maat J., Dijkema R. Comparison of nucleotide sequences of the early E1a regions for subgroups A, B and C of human adenoviruses. Gene. 1980 Dec;12(1-2):63–76. doi: 10.1016/0378-1119(80)90016-5. [DOI] [PubMed] [Google Scholar]