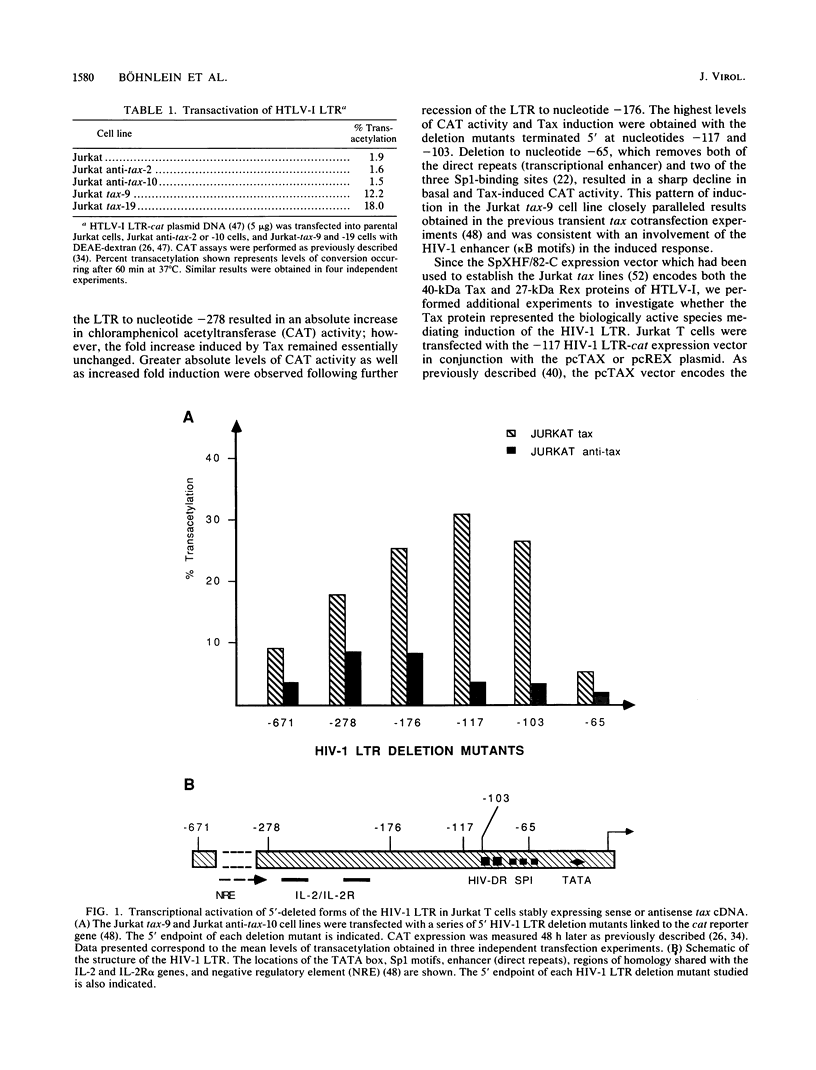
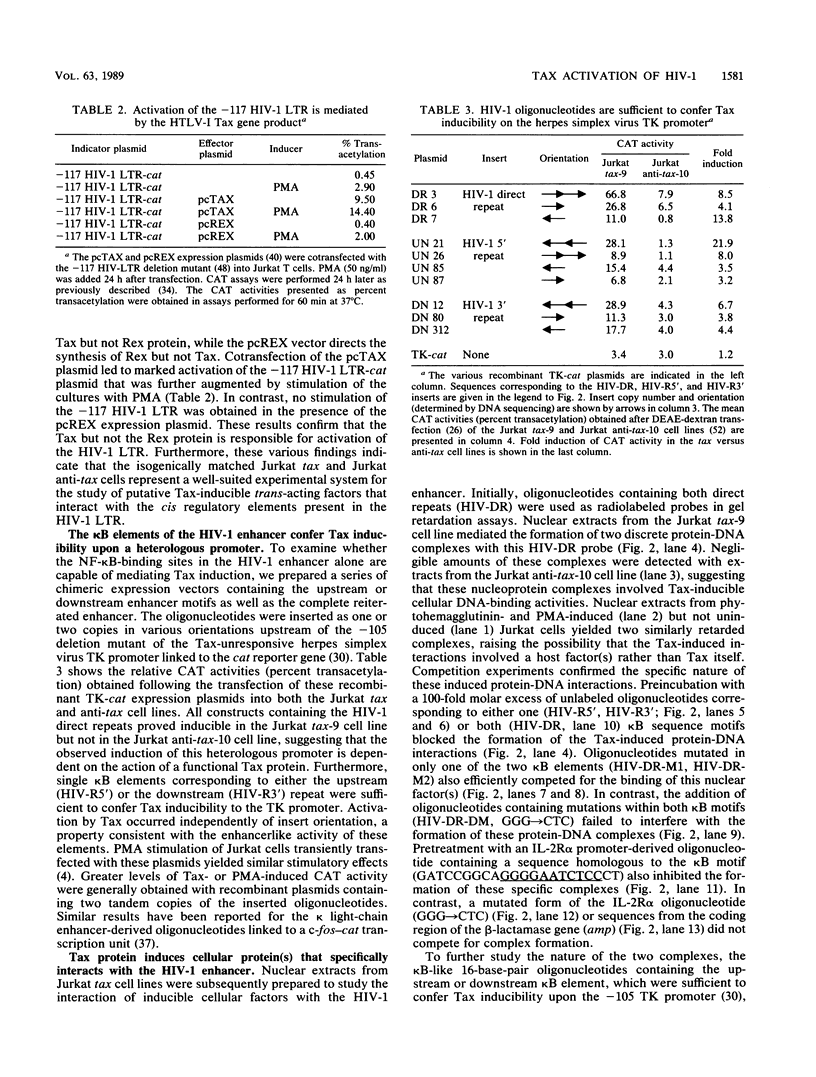
Abstract
The human immunodeficiency virus type 1 (HIV-1) preferentially infects CD4+ T lymphocytes and may exist as a latent provirus within these cells for extended periods. The transition to a productive retroviral infection results in T-cell death and clinically may lead to the acquired immune deficiency syndrome. Accelerated production of infectious HIV-1 virions appears to be closely linked to a heightened state of T-cell activation. The transactivator (Tax) protein of the type I human T-cell leukemia virus (HTLV-I) can produce such an activated T-cell phenotype and augments activity of the HIV-1 long terminal repeat. One Tax-responsive region within the HIV-1 long terminal repeat has been mapped to a locus composed of two 10-base-pair direct repeats sharing homology with the binding site for the eucaryotic transcription factor NF-kappaB (GGGACTTTCC). Tax-expressing Jurkat T cells contain one or more inducible cellular proteins that specifically associate with the HIV-1 enhancer at these binding sites. Microscale DNA affinity precipitation assays identified a Tax-inducible 86-kilodalton protein, HIVEN86A, as one of these HIV-1 enhancer-binding factors. The interaction of HIVEN86A, and presumably other cellular proteins, with the HIV-1 enhancer appears functionally important as oligonucleotides corresponding to this enhancer were sufficient to impart Tax inducibility to an unresponsive heterologous promoter. These findings suggest that the Tax-inducible cellular protein HIVEN86A plays an important role in the transcriptional activation of the HIV-1 enhancer. These specific protein-DNA interactions may also be important for the transition of HIV-1 from a latent to a productive mode of infection. Furthermore, these findings highlight an intriguing biological interplay between HTLV-1 and HIV-1 through a cellular transcriptional pathway that is normally involved in T-cell activation and growth.
Full text
PDF

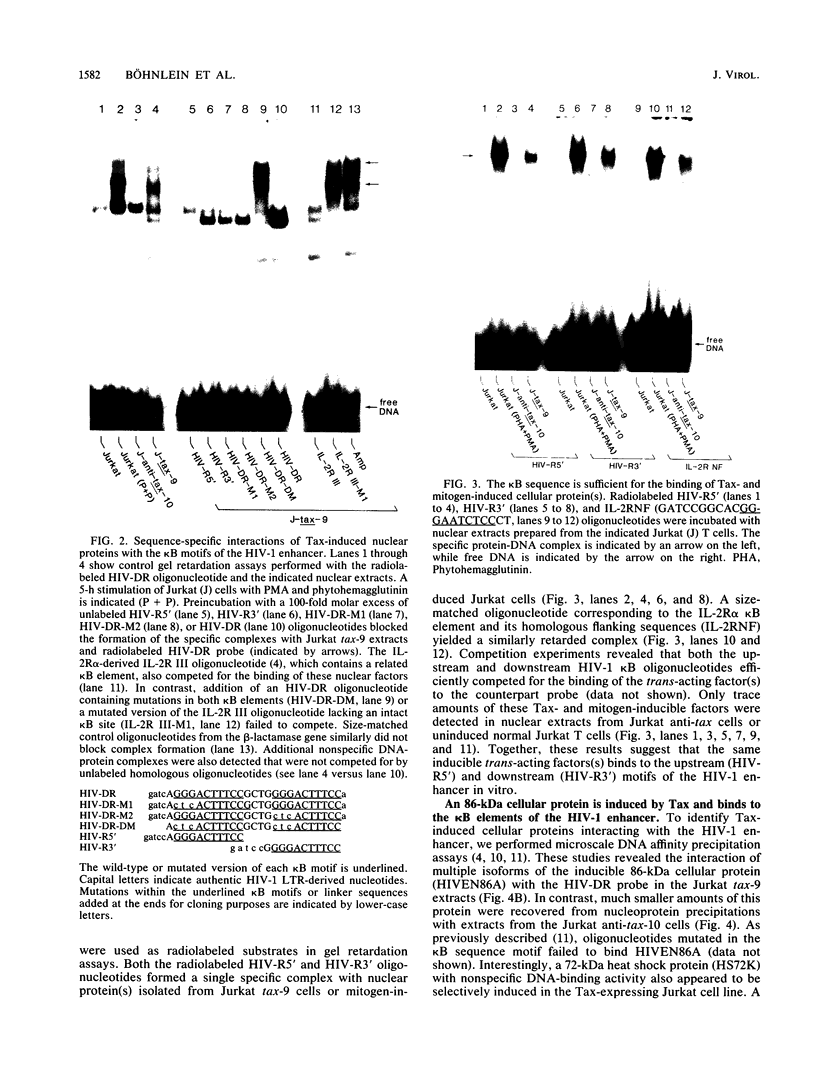
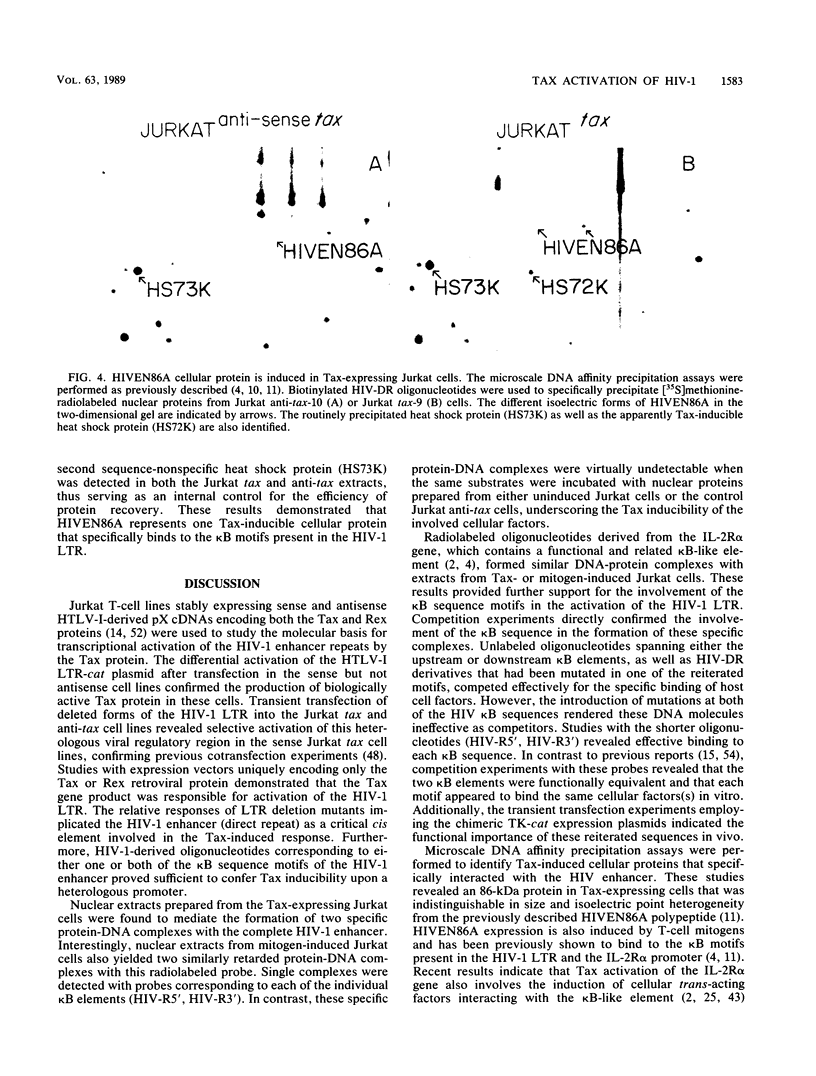



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arya S. K., Guo C., Josephs S. F., Wong-Staal F. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Jul 5;229(4708):69–73. doi: 10.1126/science.2990040. [DOI] [PubMed] [Google Scholar]
- Ballard D. W., Böhnlein E., Lowenthal J. W., Wano Y., Franza B. R., Greene W. C. HTLV-I tax induces cellular proteins that activate the kappa B element in the IL-2 receptor alpha gene. Science. 1988 Sep 23;241(4873):1652–1655. doi: 10.1126/science.241.4873.1652. [DOI] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Böhnlein E., Lowenthal J. W., Siekevitz M., Ballard D. W., Franza B. R., Greene W. C. The same inducible nuclear proteins regulates mitogen activation of both the interleukin-2 receptor-alpha gene and type 1 HIV. Cell. 1988 Jun 3;53(5):827–836. doi: 10.1016/0092-8674(88)90099-2. [DOI] [PubMed] [Google Scholar]
- Cross S. L., Feinberg M. B., Wolf J. B., Holbrook N. J., Wong-Staal F., Leonard W. J. Regulation of the human interleukin-2 receptor alpha chain promoter: activation of a nonfunctional promoter by the transactivator gene of HTLV-I. Cell. 1987 Apr 10;49(1):47–56. doi: 10.1016/0092-8674(87)90754-9. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Trans-activation of human immunodeficiency virus occurs via a bimodal mechanism. Cell. 1986 Sep 26;46(7):973–982. doi: 10.1016/0092-8674(86)90696-3. [DOI] [PubMed] [Google Scholar]
- Curran J. W., Morgan W. M., Hardy A. M., Jaffe H. W., Darrow W. W., Dowdle W. R. The epidemiology of AIDS: current status and future prospects. Science. 1985 Sep 27;229(4720):1352–1357. doi: 10.1126/science.2994217. [DOI] [PubMed] [Google Scholar]
- Dinter H., Chiu R., Imagawa M., Karin M., Jones K. A. In vitro activation of the HIV-1 enhancer in extracts from cells treated with a phorbol ester tumor promoter. EMBO J. 1987 Dec 20;6(13):4067–4071. doi: 10.1002/j.1460-2075.1987.tb02752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folks T., Powell D. M., Lightfoote M. M., Benn S., Martin M. A., Fauci A. S. Induction of HTLV-III/LAV from a nonvirus-producing T-cell line: implications for latency. Science. 1986 Feb 7;231(4738):600–602. doi: 10.1126/science.3003906. [DOI] [PubMed] [Google Scholar]
- Franza B. R., Jr, Josephs S. F., Gilman M. Z., Ryan W., Clarkson B. Characterization of cellular proteins recognizing the HIV enhancer using a microscale DNA-affinity precipitation assay. 1987 Nov 26-Dec 2Nature. 330(6146):391–395. doi: 10.1038/330391a0. [DOI] [PubMed] [Google Scholar]
- Fujisawa J., Seiki M., Sato M., Yoshida M. A transcriptional enhancer sequence of HTLV-I is responsible for trans-activation mediated by p40 chi HTLV-I. EMBO J. 1986 Apr;5(4):713–718. doi: 10.1002/j.1460-2075.1986.tb04272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Gallo R., Wong-Staal F., Montagnier L., Haseltine W. A., Yoshida M. HIV/HTLV gene nomenclature. Nature. 1988 Jun 9;333(6173):504–504. doi: 10.1038/333504a0. [DOI] [PubMed] [Google Scholar]
- Garcia J. A., Wu F. K., Mitsuyasu R., Gaynor R. B. Interactions of cellular proteins involved in the transcriptional regulation of the human immunodeficiency virus. EMBO J. 1987 Dec 1;6(12):3761–3770. doi: 10.1002/j.1460-2075.1987.tb02711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzolo L., Duc Dodon M. Direct activation of resting T lymphocytes by human T-lymphotropic virus type I. Nature. 1987 Apr 16;326(6114):714–717. doi: 10.1038/326714a0. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Phelps W., Feigenbaum L., Ostrove J. M., Adachi A., Howley P. M., Khoury G., Ginsberg H. S., Martin M. A. Trans-activation of the human immunodeficiency virus long terminal repeat sequence by DNA viruses. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9759–9763. doi: 10.1073/pnas.83.24.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene W. C., Leonard W. J., Depper J. M. Growth of human T lymphocytes: an analysis of interleukin 2 and its cellular receptor. Prog Hematol. 1986;14:283–301. [PubMed] [Google Scholar]
- Hidaka M., Inoue J., Yoshida M., Seiki M. Post-transcriptional regulator (rex) of HTLV-1 initiates expression of viral structural proteins but suppresses expression of regulatory proteins. EMBO J. 1988 Feb;7(2):519–523. doi: 10.1002/j.1460-2075.1988.tb02840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue J., Seiki M., Taniguchi T., Tsuru S., Yoshida M. Induction of interleukin 2 receptor gene expression by p40x encoded by human T-cell leukemia virus type 1. EMBO J. 1986 Nov;5(11):2883–2888. doi: 10.1002/j.1460-2075.1986.tb04583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue J., Yoshida M., Seiki M. Transcriptional (p40x) and post-transcriptional (p27x-III) regulators are required for the expression and replication of human T-cell leukemia virus type I genes. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3653–3657. doi: 10.1073/pnas.84.11.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Luciw P. A., Tjian R. Activation of the AIDS retrovirus promoter by the cellular transcription factor, Sp1. Science. 1986 May 9;232(4751):755–759. doi: 10.1126/science.3008338. [DOI] [PubMed] [Google Scholar]
- Kaufman J. D., Valandra G., Roderiquez G., Bushar G., Giri C., Norcross M. A. Phorbol ester enhances human immunodeficiency virus-promoted gene expression and acts on a repeated 10-base-pair functional enhancer element. Mol Cell Biol. 1987 Oct;7(10):3759–3766. doi: 10.1128/mcb.7.10.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K., Scheidereit C., Roeder R. G. Identification and purification of a human immunoglobulin-enhancer-binding protein (NF-kappa B) that activates transcription from a human immunodeficiency virus type 1 promoter in vitro. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4700–4704. doi: 10.1073/pnas.85.13.4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K., Nabel G. J. HTLV-1 transactivator induces interleukin-2 receptor expression through an NF-kappa B-like factor. Nature. 1988 Jun 23;333(6175):776–778. doi: 10.1038/333776a0. [DOI] [PubMed] [Google Scholar]
- Lowenthal J. W., Böhnlein E., Ballard D. W., Greene W. C. Regulation of interleukin 2 receptor alpha subunit (Tac or CD25 antigen) gene expression: binding of inducible nuclear proteins to discrete promoter sequences correlates with transcriptional activation. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4468–4472. doi: 10.1073/pnas.85.12.4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama M., Shibuya H., Harada H., Hatakeyama M., Seiki M., Fujita T., Inoue J., Yoshida M., Taniguchi T. Evidence for aberrant activation of the interleukin-2 autocrine loop by HTLV-1-encoded p40x and T3/Ti complex triggering. Cell. 1987 Jan 30;48(2):343–350. doi: 10.1016/0092-8674(87)90437-5. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Gavis E. R., Kingsbury R., Axel R. Analysis of transcriptional regulatory signals of the HSV thymidine kinase gene: identification of an upstream control region. Cell. 1981 Aug;25(2):385–398. doi: 10.1016/0092-8674(81)90057-x. [DOI] [PubMed] [Google Scholar]
- Miksicek R., Heber A., Schmid W., Danesch U., Posseckert G., Beato M., Schütz G. Glucocorticoid responsiveness of the transcriptional enhancer of Moloney murine sarcoma virus. Cell. 1986 Jul 18;46(2):283–290. doi: 10.1016/0092-8674(86)90745-2. [DOI] [PubMed] [Google Scholar]
- Mosca J. D., Bednarik D. P., Raj N. B., Rosen C. A., Sodroski J. G., Haseltine W. A., Hayward G. S., Pitha P. M. Activation of human immunodeficiency virus by herpesvirus infection: identification of a region within the long terminal repeat that responds to a trans-acting factor encoded by herpes simplex virus 1. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7408–7412. doi: 10.1073/pnas.84.21.7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca J. D., Bednarik D. P., Raj N. B., Rosen C. A., Sodroski J. G., Haseltine W. A., Pitha P. M. Herpes simplex virus type-1 can reactivate transcription of latent human immunodeficiency virus. Nature. 1987 Jan 1;325(6099):67–70. doi: 10.1038/325067a0. [DOI] [PubMed] [Google Scholar]
- Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987 Apr 16;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Paskalis H., Felber B. K., Pavlakis G. N. Cis-acting sequences responsible for the transcriptional activation of human T-cell leukemia virus type I constitute a conditional enhancer. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6558–6562. doi: 10.1073/pnas.83.17.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterlin B. M., Luciw P. A., Barr P. J., Walker M. D. Elevated levels of mRNA can account for the trans-activation of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9734–9738. doi: 10.1073/pnas.83.24.9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. W., Lenardo M., Baltimore D. Oligonucleotide that binds nuclear factor NF-kappa B acts as a lymphoid-specific and inducible enhancer element. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1482–1486. doi: 10.1073/pnas.85.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Reitz M. S., Kalyanaraman V. S., Gallo R. C. Isolation of a new type C retrovirus (HTLV) in primary uncultured cells of a patient with Sézary T-cell leukaemia. Nature. 1981 Nov 19;294(5838):268–271. doi: 10.1038/294268a0. [DOI] [PubMed] [Google Scholar]
- Rimsky L., Hauber J., Dukovich M., Malim M. H., Langlois A., Cullen B. R., Greene W. C. Functional replacement of the HIV-1 rev protein by the HTLV-1 rex protein. Nature. 1988 Oct 20;335(6192):738–740. doi: 10.1038/335738a0. [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Park R., Sodroski J. G., Haseltine W. A. Multiple sequence elements are required for regulation of human T-cell leukemia virus gene expression. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4919–4923. doi: 10.1073/pnas.84.14.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Haseltine W. A. The location of cis-acting regulatory sequences in the human T cell lymphotropic virus type III (HTLV-III/LAV) long terminal repeat. Cell. 1985 Jul;41(3):813–823. doi: 10.1016/s0092-8674(85)80062-3. [DOI] [PubMed] [Google Scholar]
- Ruben S., Poteat H., Tan T. H., Kawakami K., Roeder R., Haseltine W., Rosen C. A. Cellular transcription factors and regulation of IL-2 receptor gene expression by HTLV-I tax gene product. Science. 1988 Jul 1;241(4861):89–92. doi: 10.1126/science.2838905. [DOI] [PubMed] [Google Scholar]
- Seiki M., Inoue J., Takeda T., Yoshida M. Direct evidence that p40x of human T-cell leukemia virus type I is a trans-acting transcriptional activator. EMBO J. 1986 Mar;5(3):561–565. doi: 10.1002/j.1460-2075.1986.tb04247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Shimotohno K., Takano M., Teruuchi T., Miwa M. Requirement of multiple copies of a 21-nucleotide sequence in the U3 regions of human T-cell leukemia virus type I and type II long terminal repeats for trans-acting activation of transcription. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8112–8116. doi: 10.1073/pnas.83.21.8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekevitz M., Feinberg M. B., Holbrook N., Wong-Staal F., Greene W. C. Activation of interleukin 2 and interleukin 2 receptor (Tac) promoter expression by the trans-activator (tat) gene product of human T-cell leukemia virus, type I. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5389–5393. doi: 10.1073/pnas.84.15.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekevitz M., Josephs S. F., Dukovich M., Peffer N., Wong-Staal F., Greene W. C. Activation of the HIV-1 LTR by T cell mitogens and the trans-activator protein of HTLV-I. Science. 1987 Dec 11;238(4833):1575–1578. doi: 10.1126/science.2825351. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Patarca R., Rosen C., Wong-Staal F., Haseltine W. Location of the trans-activating region on the genome of human T-cell lymphotropic virus type III. Science. 1985 Jul 5;229(4708):74–77. doi: 10.1126/science.2990041. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Tong-Starksen S. E., Luciw P. A., Peterlin B. M. Human immunodeficiency virus long terminal repeat responds to T-cell activation signals. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6845–6849. doi: 10.1073/pnas.84.19.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wano Y., Feinberg M., Hosking J. B., Bogerd H., Greene W. C. Stable expression of the tax gene of type I human T-cell leukemia virus in human T cells activates specific cellular genes involved in growth. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9733–9737. doi: 10.1073/pnas.85.24.9733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Staal F., Gallo R. C. Human T-lymphotropic retroviruses. Nature. 1985 Oct 3;317(6036):395–403. doi: 10.1038/317395a0. [DOI] [PubMed] [Google Scholar]
- Wu F. K., Garcia J. A., Harrich D., Gaynor R. B. Purification of the human immunodeficiency virus type 1 enhancer and TAR binding proteins EBP-1 and UBP-1. EMBO J. 1988 Jul;7(7):2117–2130. doi: 10.1002/j.1460-2075.1988.tb03051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Garcia J., Mitsuyasu R., Gaynor R. Alterations in binding characteristics of the human immunodeficiency virus enhancer factor. J Virol. 1988 Jan;62(1):218–225. doi: 10.1128/jvi.62.1.218-225.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack J. A., Cann A. J., Lugo J. P., Chen I. S. HIV-1 production from infected peripheral blood T cells after HTLV-I induced mitogenic stimulation. Science. 1988 May 20;240(4855):1026–1029. doi: 10.1126/science.2835813. [DOI] [PubMed] [Google Scholar]
- Zagury D., Bernard J., Leonard R., Cheynier R., Feldman M., Sarin P. S., Gallo R. C. Long-term cultures of HTLV-III--infected T cells: a model of cytopathology of T-cell depletion in AIDS. Science. 1986 Feb 21;231(4740):850–853. doi: 10.1126/science.2418502. [DOI] [PubMed] [Google Scholar]