Abstract
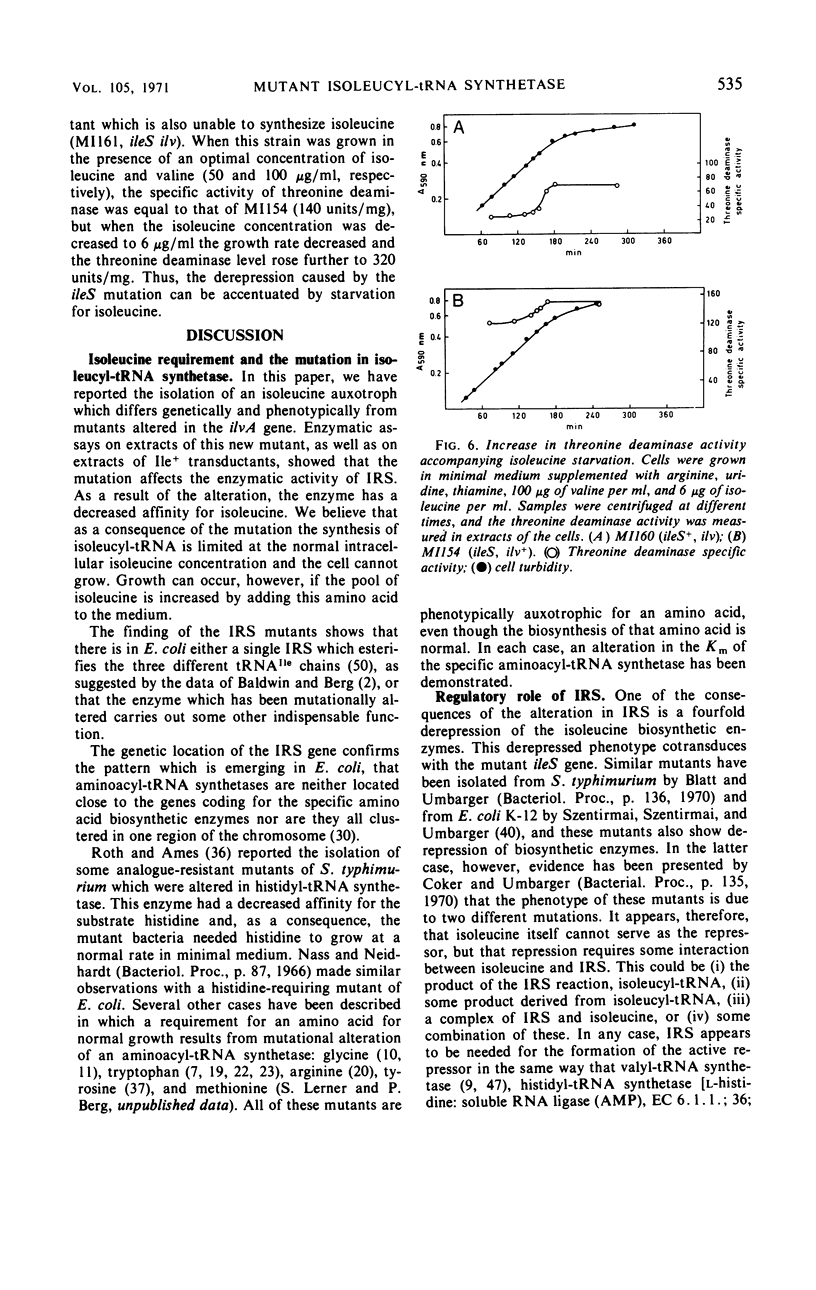
Among mutants which require isoleucine, but not valine, for growth, we have found two distinguishable classes. One is defective in the biosynthetic enzyme threonine deaminase (l-threonine hydro-lyase, deaminating, EC 4.2.1.16) and the other has an altered isoleucyl transfer ribonucleic acid (tRNA) synthetase [l-isoleucine: soluble RNA ligase (adenosine monophosphate), EC 6.1.1.5]. The mutation which affects ileS, the structural gene for isoleucyl-tRNA synthetase, is located between thr and pyrA at 0 min on the map of the Escherichia coli chromosome. This mutationally altered isoleucyl-tRNA synthetase has an apparent Km for isoleucine (∼1 mm) 300-fold higher than that of the enzyme from wild type; on the other hand, the apparent Vmax is altered only slightly. When the mutationally altered ileS allele was introduced into a strain which overproduces isoleucine, the resulting strain could grow without addition of isoleucine. We conclude that the normal intracellular isoleucine level is not high enough to allow efficient charging to tRNAIle by the mutant enzyme because of the Km defect. A consequence of the alteration in isoleucyl-tRNA synthetase was a fourfold derepression of the enzymes responsible for isoleucine biosynthesis. Thus, a functional isoleucyl-tRNA synthetase is needed for isoleucine to act as a regulator of its own biosynthesis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt D. J., Berg P. Isoleucyl transfer ribonucleic acid synthetase is a single polypeptide chain. J Biol Chem. 1970 Feb 10;245(3):665–667. [PubMed] [Google Scholar]
- Baldwin A. N., Berg P. Purification and properties of isoleucyl ribonucleic acid synthetase from Escherichia coli. J Biol Chem. 1966 Feb 25;241(4):831–838. [PubMed] [Google Scholar]
- Baldwin A. N., Berg P. Transfer ribonucleic acid-induced hydrolysis of valyladenylate bound to isoleucyl ribonucleic acid synthetase. J Biol Chem. 1966 Feb 25;241(4):839–845. [PubMed] [Google Scholar]
- Burns R. O., Zarlengo M. H. Threonine deaminase from Salmonella typhimurium. I. Purification and properties. J Biol Chem. 1968 Jan 10;243(1):178–185. [PubMed] [Google Scholar]
- Doolittle W. F., Yanofsky C. Mutants of Escherichia coli with an altered tryptophanyl-transfer ribonucleic acid synthetase. J Bacteriol. 1968 Apr;95(4):1283–1294. doi: 10.1128/jb.95.4.1283-1294.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer S. B., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XVI. Pattern of multivalent repression in strain K-12. J Bacteriol. 1968 May;95(5):1680–1684. doi: 10.1128/jb.95.5.1680-1684.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EIDLIC L., NEIDHARDT F. C. ROLE OF VALYL-SRNA SYNTHETASE IN ENZYME REPRESSION. Proc Natl Acad Sci U S A. 1965 Mar;53:539–543. doi: 10.1073/pnas.53.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUNDLICH M., BURNS R. O., UMBARGER H. E. Control of isoleucine, valine, and leucine biosynthesis. I. Multivalent repression. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1804–1808. doi: 10.1073/pnas.48.10.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk W. R., Berg P. Characterization of altered forms of glycyl transfer ribonucleic acid synthetase and the effects of such alterations on aminoacyl transfer ribonucleic acid synthesis in vivo. J Bacteriol. 1970 Apr;102(1):204–212. doi: 10.1128/jb.102.1.204-212.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk W. R., Berg P. Isolation and partial characterization of Escherichia coli mutants with altered glycyl transfer ribonucleic acid synthetases. J Bacteriol. 1970 Apr;102(1):193–203. doi: 10.1128/jb.102.1.193-203.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORINI L., KAUFMAN H. Selecting bacterial mutants by the penicillin method. Science. 1960 Feb 26;131(3400):604–605. doi: 10.1126/science.131.3400.604. [DOI] [PubMed] [Google Scholar]
- Groves W. E., Davis F. C., Jr, Sells B. H. Spectrophotometric determination of microgram quantities of protein without nucleic acid interference. Anal Biochem. 1968 Feb;22(2):195–210. doi: 10.1016/0003-2697(68)90307-2. [DOI] [PubMed] [Google Scholar]
- Hill C. W., Foulds J., Soll L., Berg P. Instability of a missense suppressor resulting from a duplication of genetic material. J Mol Biol. 1969 Feb 14;39(3):563–581. doi: 10.1016/0022-2836(69)90146-6. [DOI] [PubMed] [Google Scholar]
- Hiraga S., Ito K., Hamada K., Yura T. A new regulatory gene for the tryptophan operon of Escherichia coli. Biochem Biophys Res Commun. 1967 Mar 9;26(5):522–527. doi: 10.1016/0006-291x(67)90095-2. [DOI] [PubMed] [Google Scholar]
- Hirshfield I. N., Horn P. C., Hopwood D. A., Maas W. K., DeDeken R. Studies on the mechanism of repression of arginine biosynthesis in Escherichia coli. 3. Repression of enzymes of arginine biosynthesis in arginyl-tRNA synthetase mutants. J Mol Biol. 1968 Jul 14;35(1):83–93. doi: 10.1016/s0022-2836(68)80038-5. [DOI] [PubMed] [Google Scholar]
- Iaccarino M., Berg P. Requirement of sulfhydryl groups for the catalytic and tRNA recognition functions of isoleucyl-tRNA synthetase. J Mol Biol. 1969 Jun 14;42(2):151–169. doi: 10.1016/0022-2836(69)90036-9. [DOI] [PubMed] [Google Scholar]
- Ito K., Hiraga S., Yura T. Tryptophanyl transfer RNA synthetase and expression of the tryptophan operon in the trpS mutants of Escherichia coli. Genetics. 1969 Mar;61(3):521–538. doi: 10.1093/genetics/61.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano Y., Matsushiro A., Shimura Y. Isolation of the novel regulatory mutants of the tryptophan biosynthetic system in Escherichia coli. Mol Gen Genet. 1968;102(1):15–26. doi: 10.1007/BF00341866. [DOI] [PubMed] [Google Scholar]
- LEAVITT R. I., UMBARGER H. E. Isoleucine and valine metabolism in Escherichia coli. XI. Valine inhibition of the growth of Escherichia coli strain K-12. J Bacteriol. 1962 Mar;83:624–630. doi: 10.1128/jb.83.3.624-630.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Low B. Formation of merodiploids in matings with a class of Rec- recipient strains of Escherichia coli K12. Proc Natl Acad Sci U S A. 1968 May;60(1):160–167. doi: 10.1073/pnas.60.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas W. K., Davis B. D. Production of an Altered Pantothenate-Synthesizing Enzyme by a Temperature-Sensitive Mutant of Escherichia Coli. Proc Natl Acad Sci U S A. 1952 Sep;38(9):785–797. doi: 10.1073/pnas.38.9.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORRIS A. T., BERG P. MECHANISM OF AMINOACYL RNA SYNTHESIS: STUDIES WITH ISOLATED AMINOACYL ADENYLATE COMPLEXES OF ISOLEUCYL RNA SYNTHETASE. Proc Natl Acad Sci U S A. 1964 Aug;52:330–337. doi: 10.1073/pnas.52.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C. Roles of amino acid activating enzymes in cellular physiology. Bacteriol Rev. 1966 Dec;30(4):701–719. doi: 10.1128/br.30.4.701-719.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITTARD J., LOUTIT J. S., ADELBERG E. A. GENE TRANSFER BY F' STRAINS OF ESCHERICHIA COLI K-12. I. DELAY IN INITIATION OF CHROMOSOME TRANSFER. J Bacteriol. 1963 Jun;85:1394–1401. doi: 10.1128/jb.85.6.1394-1401.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. 3. MAP ORDER OF THE STRUCTURAL GENES AND OPERATOR GENES. J Bacteriol. 1965 Mar;89:661–664. doi: 10.1128/jb.89.3.661-664.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. I. GENETIC DEREPRESSION OF ENZYME FORMATION. J Bacteriol. 1964 Mar;87:566–573. doi: 10.1128/jb.87.3.566-573.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. II. IDENTIFICATION OF TWO OPERATOR GENES. J Bacteriol. 1965 Mar;89:654–660. doi: 10.1128/jb.89.3.654-660.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. R., Ames B. N. Histidine regulatory mutants in Salmonella typhimurium II. Histidine regulatory mutants having altered histidyl-tRNA synthetase. J Mol Biol. 1966 Dec 28;22(2):325–333. doi: 10.1016/0022-2836(66)90135-5. [DOI] [PubMed] [Google Scholar]
- STACEY K. A., SIMSON E. IMPROVED METHOD FOR THE ISOLATION OF THYMINE-REQUIRING MUTANTS OF ESCHERICHIA COLI. J Bacteriol. 1965 Aug;90:554–555. doi: 10.1128/jb.90.2.554-555.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger S., Nester E. W. Mutants of Escherichia coli with an altered tyrosyl-transfer ribonucleic acid synthetase. J Bacteriol. 1969 Oct;100(1):167–175. doi: 10.1128/jb.100.1.167-175.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentirmai A., Szentirmai M., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XV. Biochemical properties of mutants resistant to thiaisoleucine. J Bacteriol. 1968 May;95(5):1672–1679. doi: 10.1128/jb.95.5.1672-1679.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Isoleucine and valine metabolism in Escherichia coli. V. Antagonism between isoleucine and valine. J Bacteriol. 1955 Aug;70(2):241–248. doi: 10.1128/jb.70.2.241-248.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbarger H. E. Regulation of amino acid metabolism. Annu Rev Biochem. 1969;38:323–370. doi: 10.1146/annurev.bi.38.070169.001543. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Yarus M., Berg P. Recognition of tRNA by aminoacyl tRNA synthetases. J Mol Biol. 1967 Sep 28;28(3):479–490. doi: 10.1016/s0022-2836(67)80098-6. [DOI] [PubMed] [Google Scholar]
- Yarus M., Berg P. Recognition of tRNA by isoleucyl-tRNA synthetase. Effect of substrates on the dynamics of tRNA-enzyme interaction. J Mol Biol. 1969 Jun 14;42(2):171–189. doi: 10.1016/0022-2836(69)90037-0. [DOI] [PubMed] [Google Scholar]
- Yegian C. D., Stent G. S. Differential aminoacylation of three species of isoleucine transfer RNA from Escherichia coli. J Mol Biol. 1969 Jan 14;39(1):59–71. doi: 10.1016/0022-2836(69)90333-7. [DOI] [PubMed] [Google Scholar]


