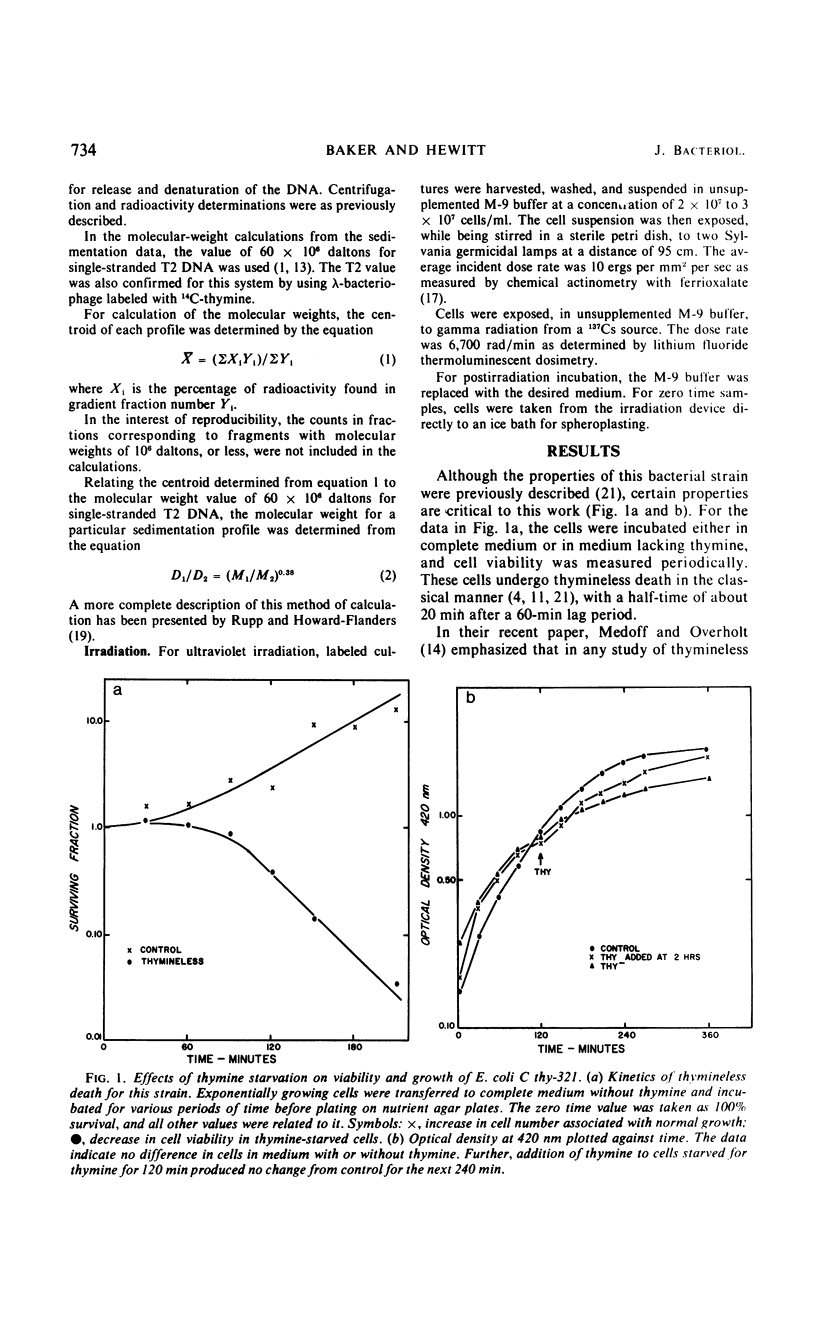
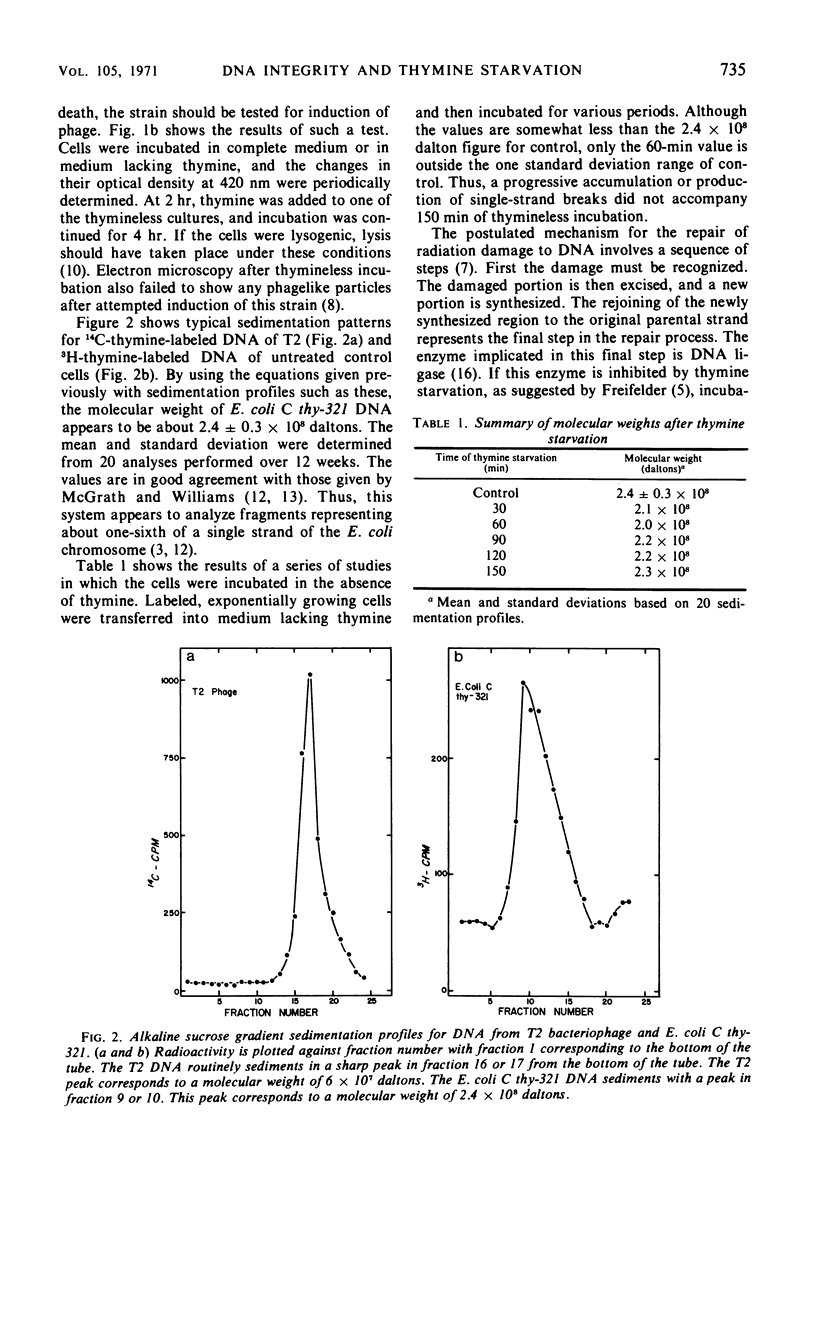
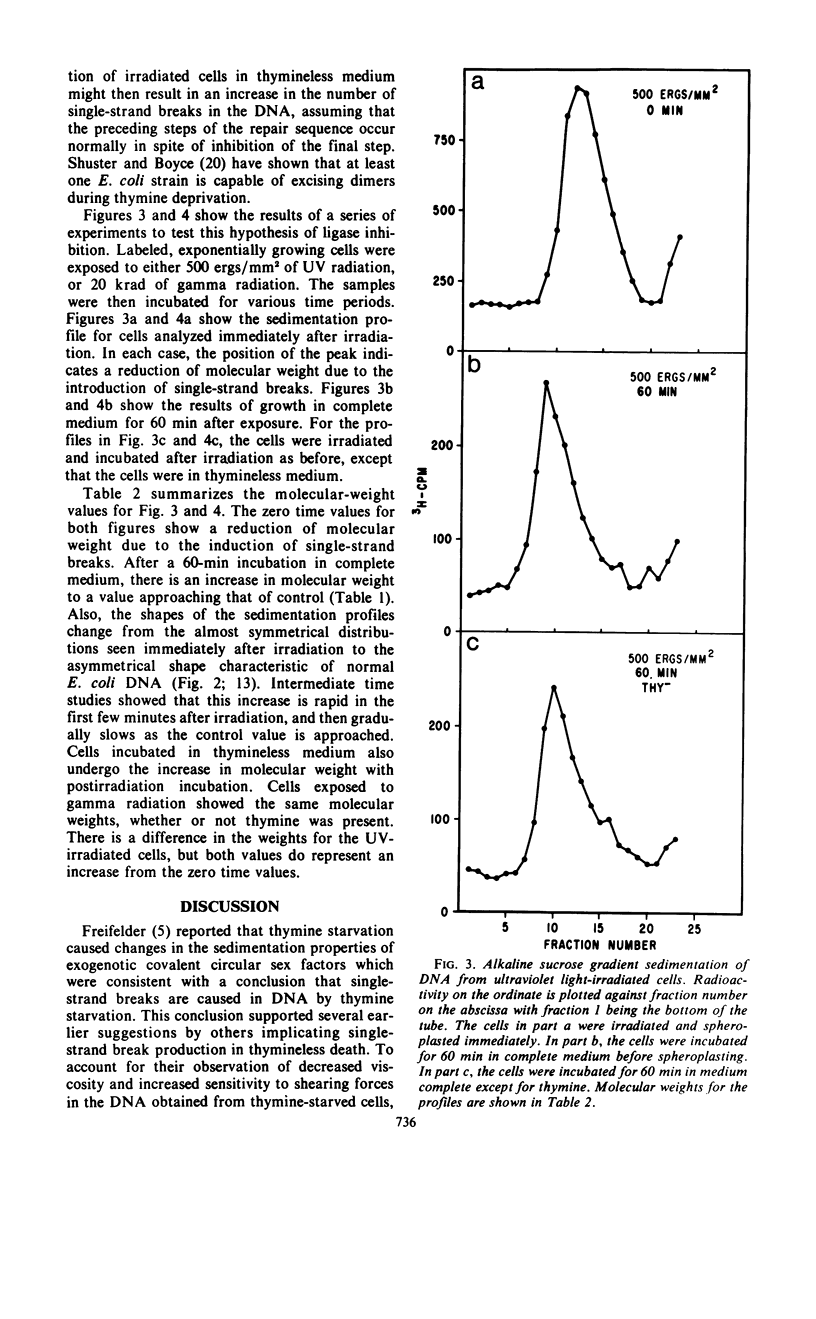
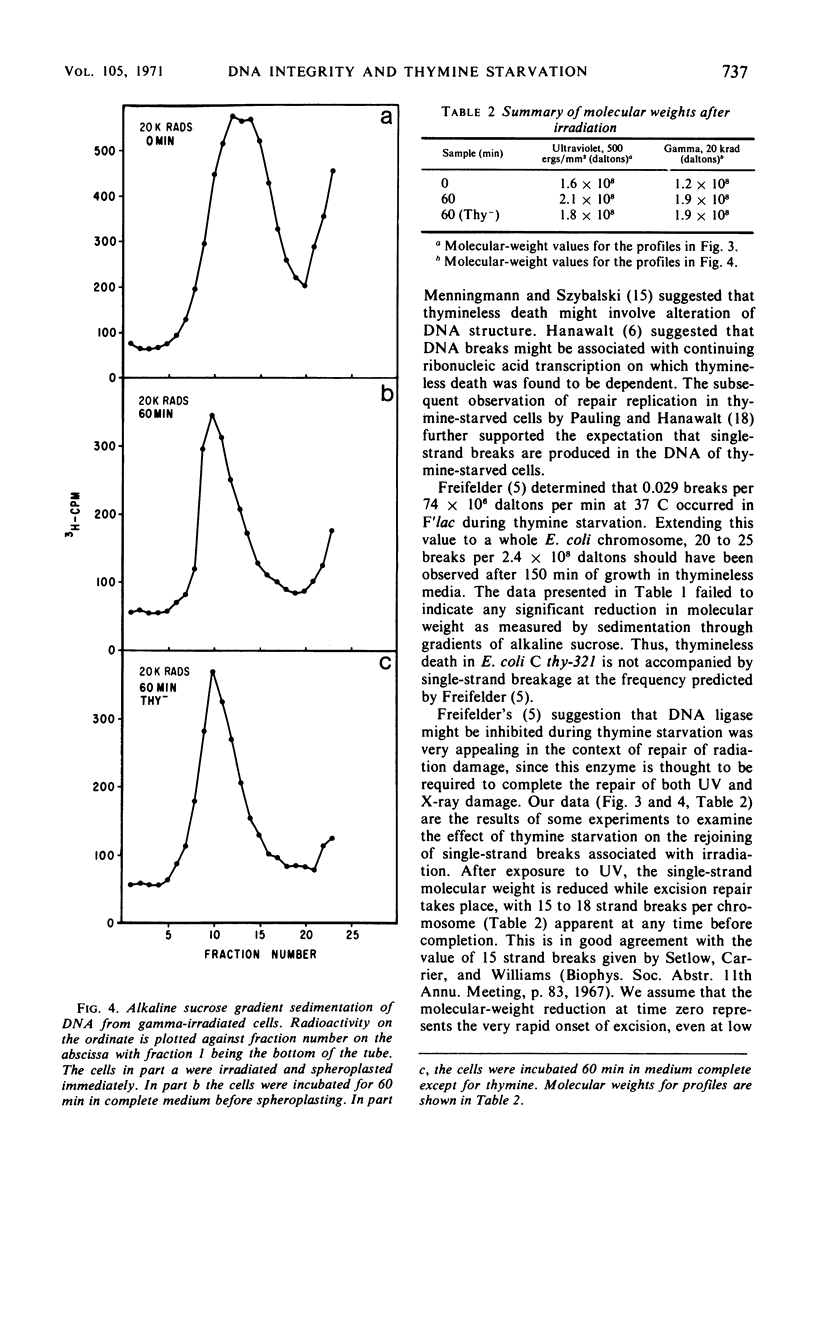
Abstract
The influence of thymine starvation on the single-strand molecular weight of deoxyribonucleic acid (DNA) from Escherichia coli was determined by sedimentation through gradients of alkaline sucrose. Growth of cells for as long as 150 min in thymineless medium did not significantly reduce the molecular weight below the control value of 2.4 ± 0.3 × 108 daltons. Incubation of cells in thymineless medium after exposure to 500 ergs/mm2 of ultraviolet light or 20 krad of 137Cs gamma rays did not appear to block the rejoining of single-strand breaks associated with irradiation. Thus, DNA repair enzymes, presumably including DNA ligase, are not significantly inhibited by thymine starvation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CAIRNS J. The bacterial chromosome and its manner of replication as seen by autoradiography. J Mol Biol. 1963 Mar;6:208–213. doi: 10.1016/s0022-2836(63)80070-4. [DOI] [PubMed] [Google Scholar]
- Cohen S. S., Barner H. D. STUDIES ON UNBALANCED GROWTH IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1954 Oct;40(10):885–893. doi: 10.1073/pnas.40.10.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D. Single-strand breaks in bacterial DNA associated with thymine starvation. J Mol Biol. 1969 Oct 14;45(1):1–7. doi: 10.1016/0022-2836(69)90205-8. [DOI] [PubMed] [Google Scholar]
- HANAWALT P. C. Involvement of synthesis of RNA in thymineless death. Nature. 1963 Apr 20;198:286–286. doi: 10.1038/198286a0. [DOI] [PubMed] [Google Scholar]
- Hewitt R., Billen D., Jorgensen G. Radiation-induced reorientation of chromosome replication sequence: generality in Escherichia coli; independence of prophage or 5-bromouracil toxicity. Radiat Res. 1967 Oct;32(2):214–226. [PubMed] [Google Scholar]
- Hewitt R., Suit J. C., Billen D. Utilization of 5-bromouracil by thymineless bacteria. J Bacteriol. 1967 Jan;93(1):86–89. doi: 10.1128/jb.93.1.86-89.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORN D., WEISSBACH A. Thymineless induction in Escherichia coli K12 (lambda). Biochim Biophys Acta. 1962 Nov 26;61:775–790. doi: 10.1016/0926-6550(62)90060-9. [DOI] [PubMed] [Google Scholar]
- MAALOE O., HANAWALT P. C. Thymine deficiency and the normal DNA replication cycle. I. J Mol Biol. 1961 Apr;3:144–155. doi: 10.1016/s0022-2836(61)80041-7. [DOI] [PubMed] [Google Scholar]
- MENNIGMANN H. D., SZYBALSKI W. Molecular mechanism of thymine-less death. Biochem Biophys Res Commun. 1962 Nov 27;9:398–404. doi: 10.1016/0006-291x(62)90023-2. [DOI] [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W. Interruptions in single strands of the DNA in slime mold and other organisms. Biophys J. 1967 May;7(3):309–317. doi: 10.1016/S0006-3495(67)86590-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W. Reconstruction in vivo of irradiated Escherichia coli deoxyribonucleic acid; the rejoining of broken pieces. Nature. 1966 Oct 29;212(5061):534–535. doi: 10.1038/212534a0. [DOI] [PubMed] [Google Scholar]
- Medoff G., Overholt S. Thymineless death in Escherichia coli 15T- and recombinants of 15T- and Escherichia coli K-12. J Bacteriol. 1970 Apr;102(1):213–216. doi: 10.1128/jb.102.1.213-216.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera B. M., Lehman I. R. Linkage of polynucleotides through phosphodiester bonds by an enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1967 May;57(5):1426–1433. doi: 10.1073/pnas.57.5.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauling C., Hanawalt P. Nonconservative DNA replication in bacteria after thymine starvation. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1728–1735. doi: 10.1073/pnas.54.6.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- Shuster R. C., Boyce R. P. The excision of thymine dimer from the DNA of UV-irradiated E. coli 15 T-A-U during thymine deprivation. Biochem Biophys Res Commun. 1964 Jul 27;16(5):489–496. doi: 10.1016/0006-291x(64)90381-x. [DOI] [PubMed] [Google Scholar]
- Suit J. C., Barbee T., Jetton S. Morphological changes in Escherichia coli strain C produced by treatments affecting deoxyribonucleic acid synthesis. J Gen Microbiol. 1967 Oct;49(1):165–173. doi: 10.1099/00221287-49-1-165. [DOI] [PubMed] [Google Scholar]
- Thomas C. A., Jr The arrangement of information in DNA molecules. J Gen Physiol. 1966 Jul;49(6):143–169. doi: 10.1085/jgp.49.6.143. [DOI] [PMC free article] [PubMed] [Google Scholar]


