Abstract
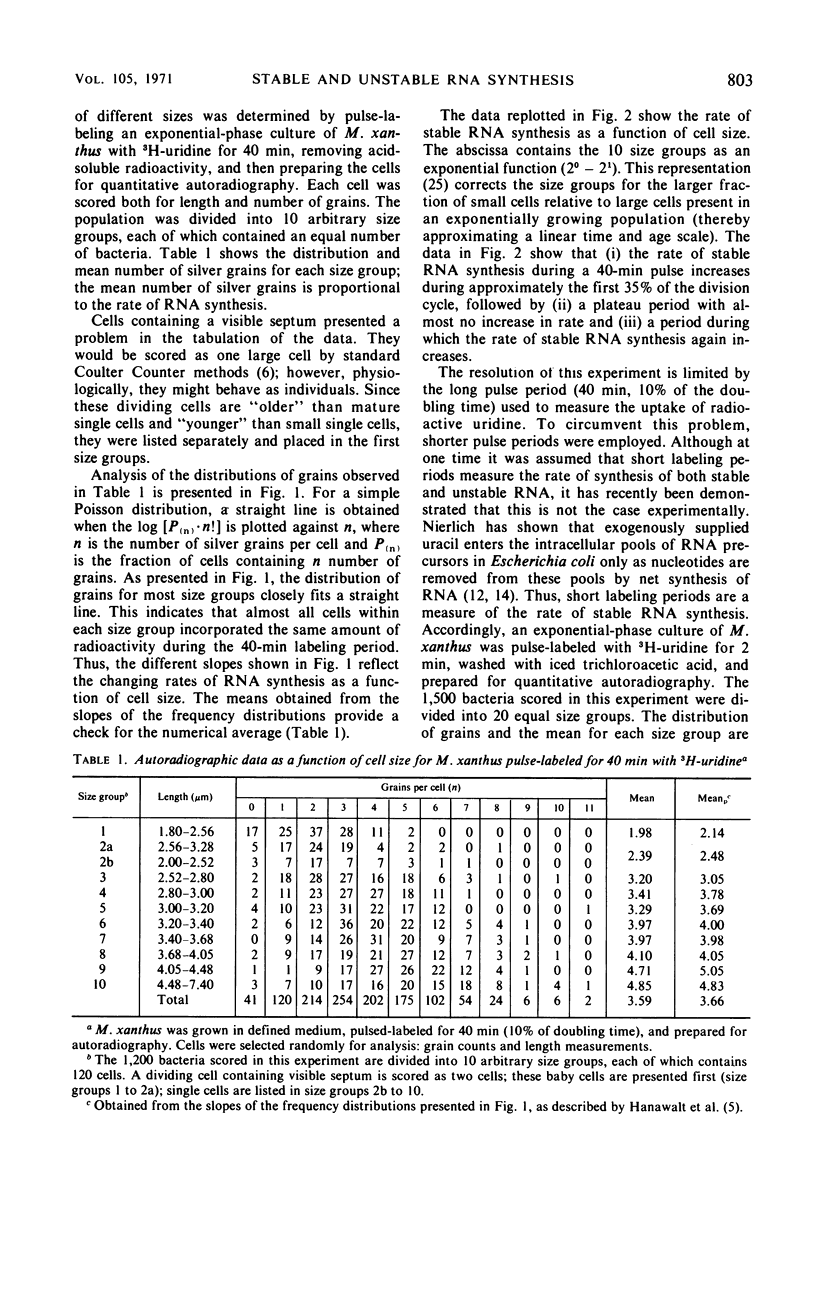
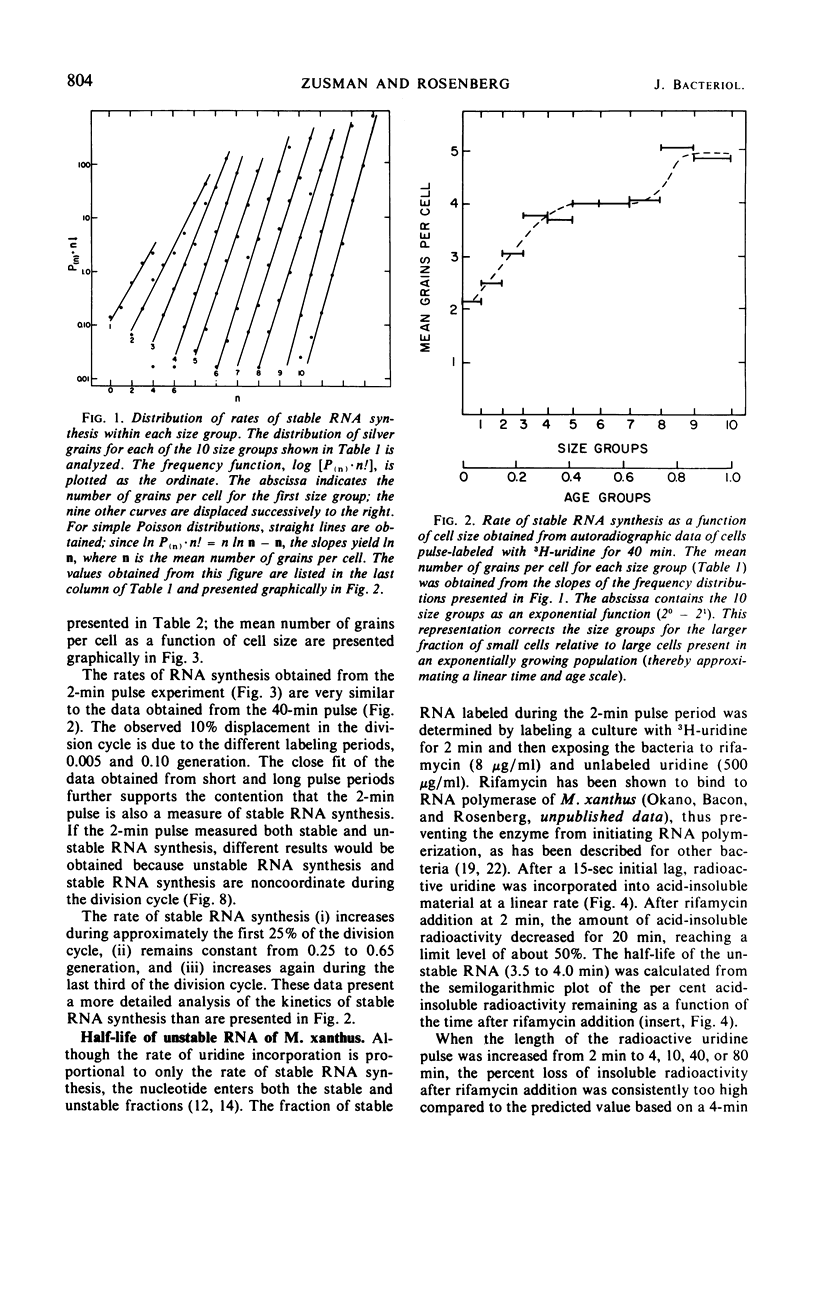
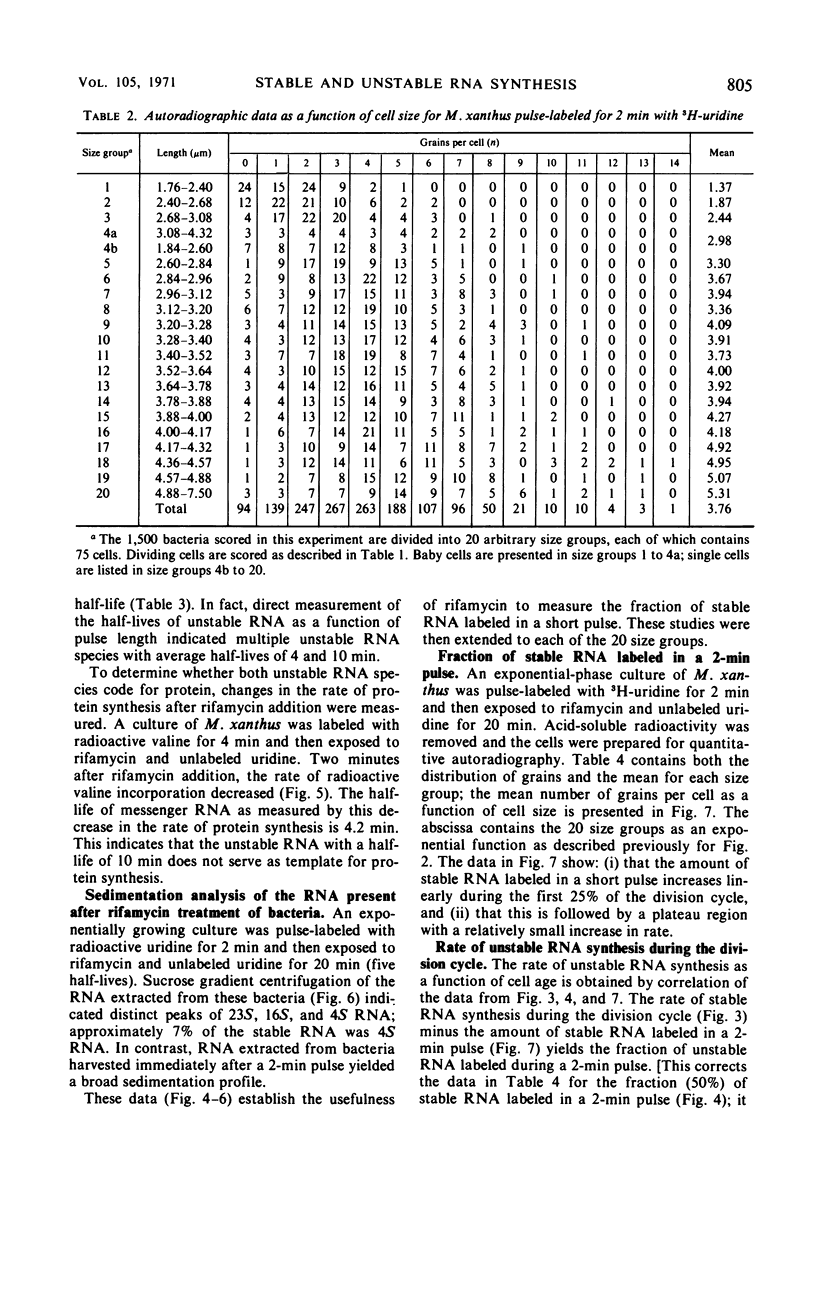
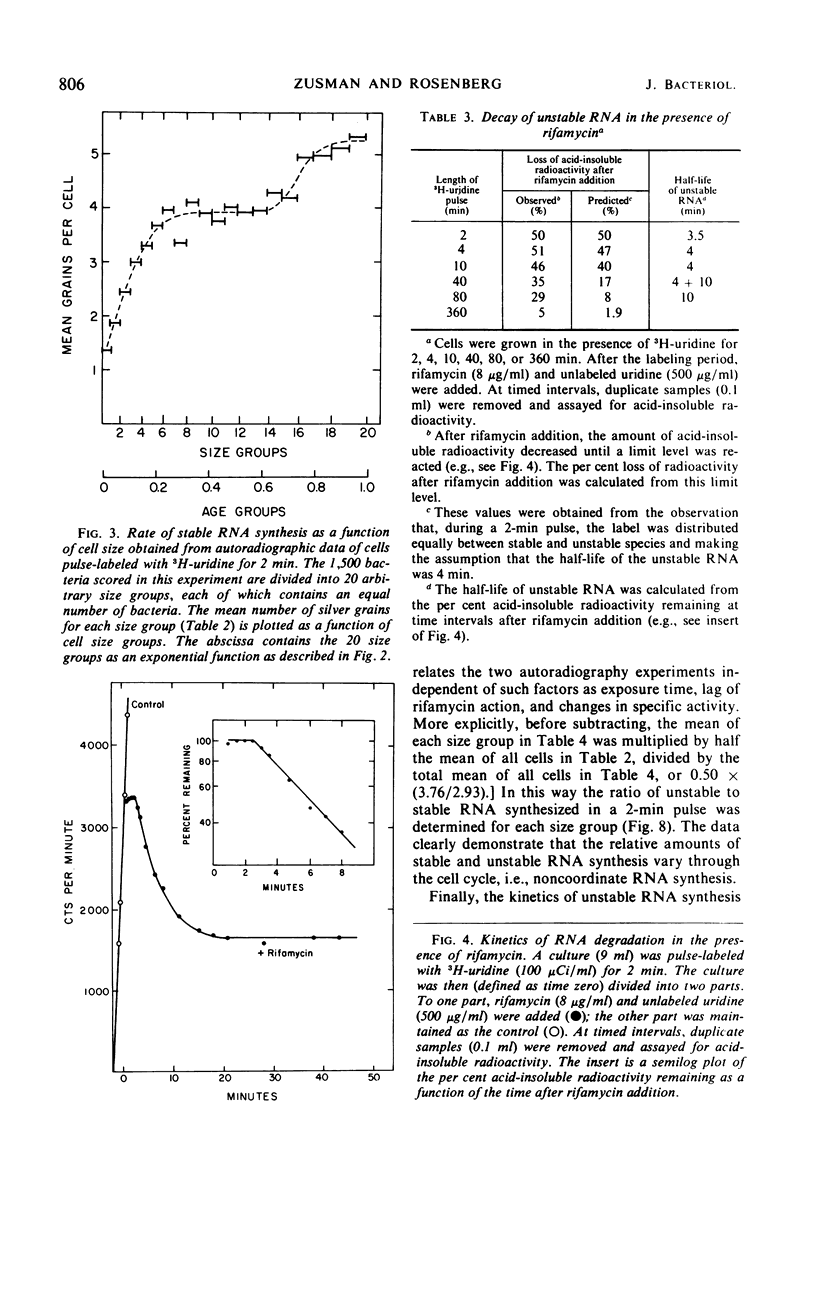
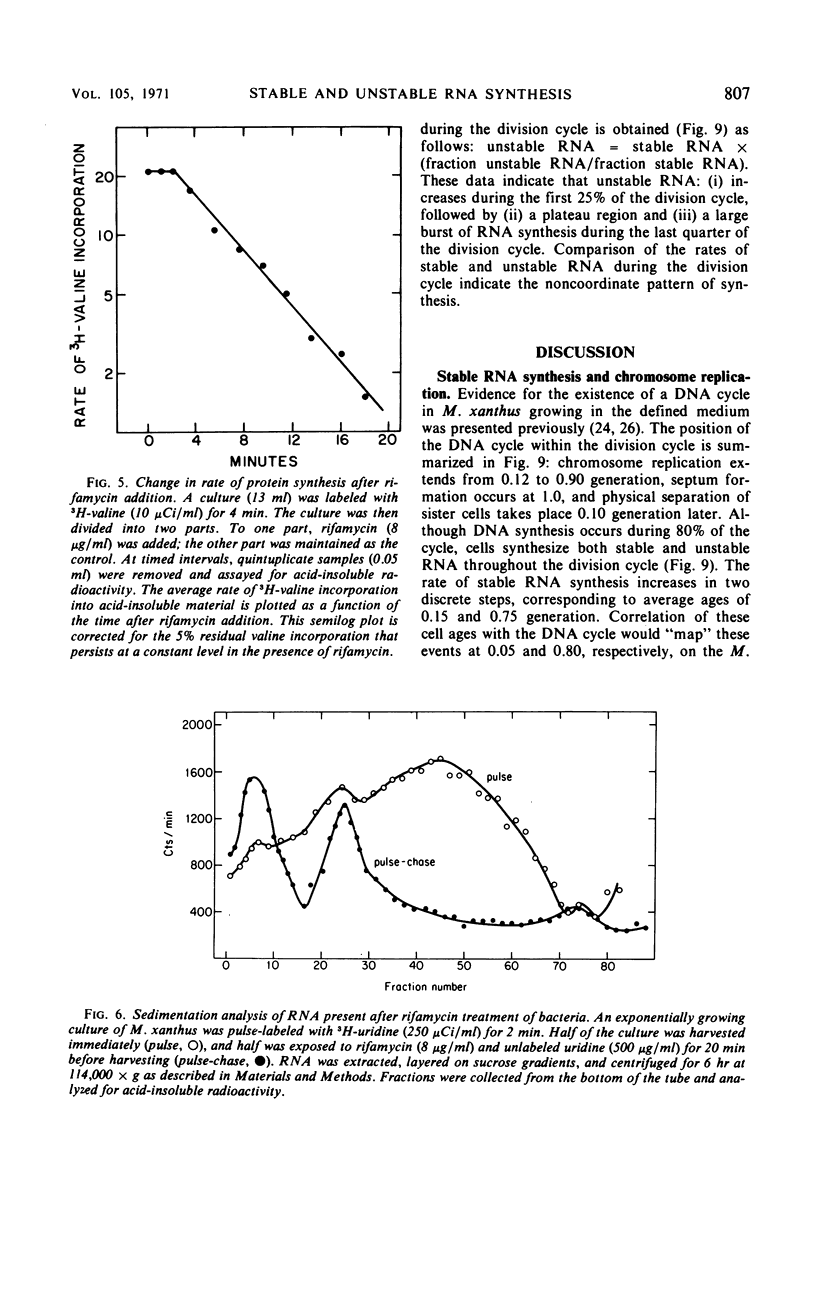
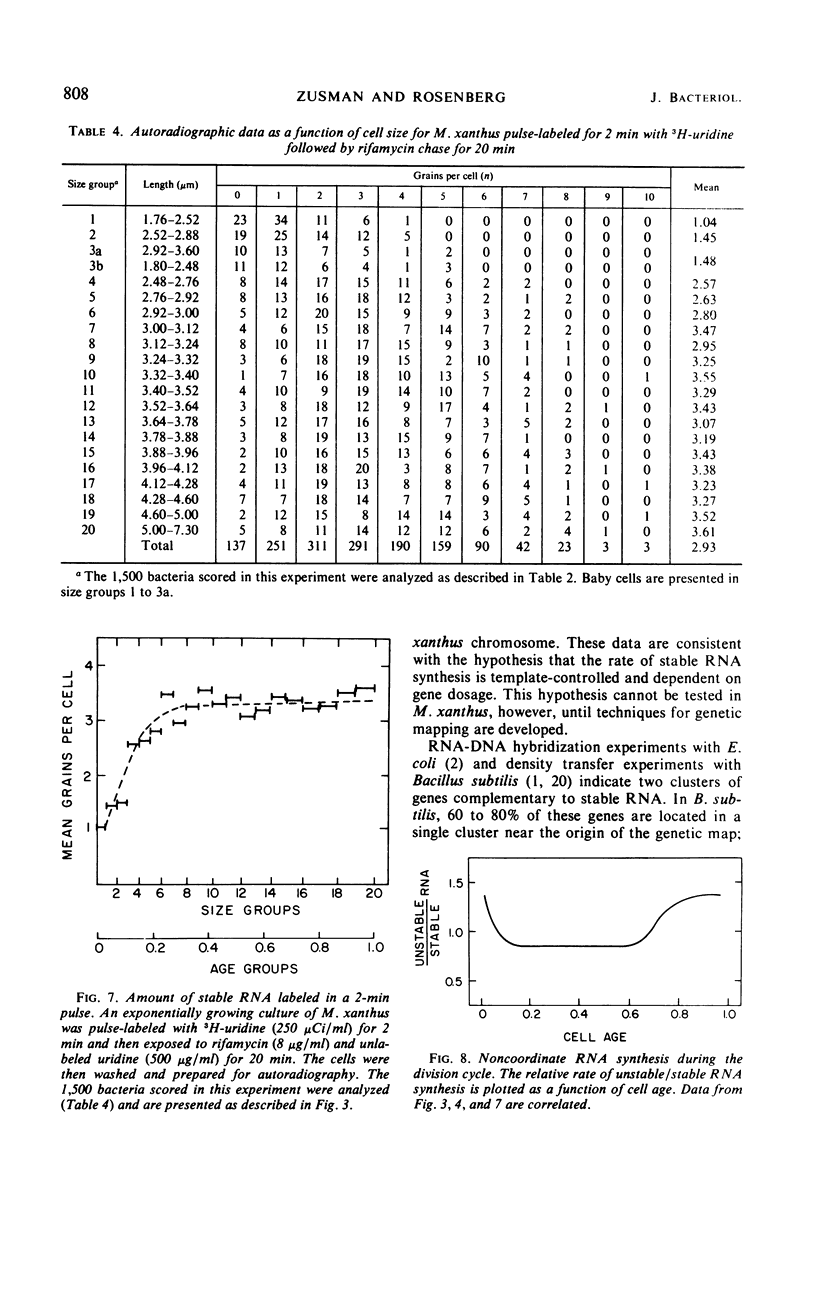
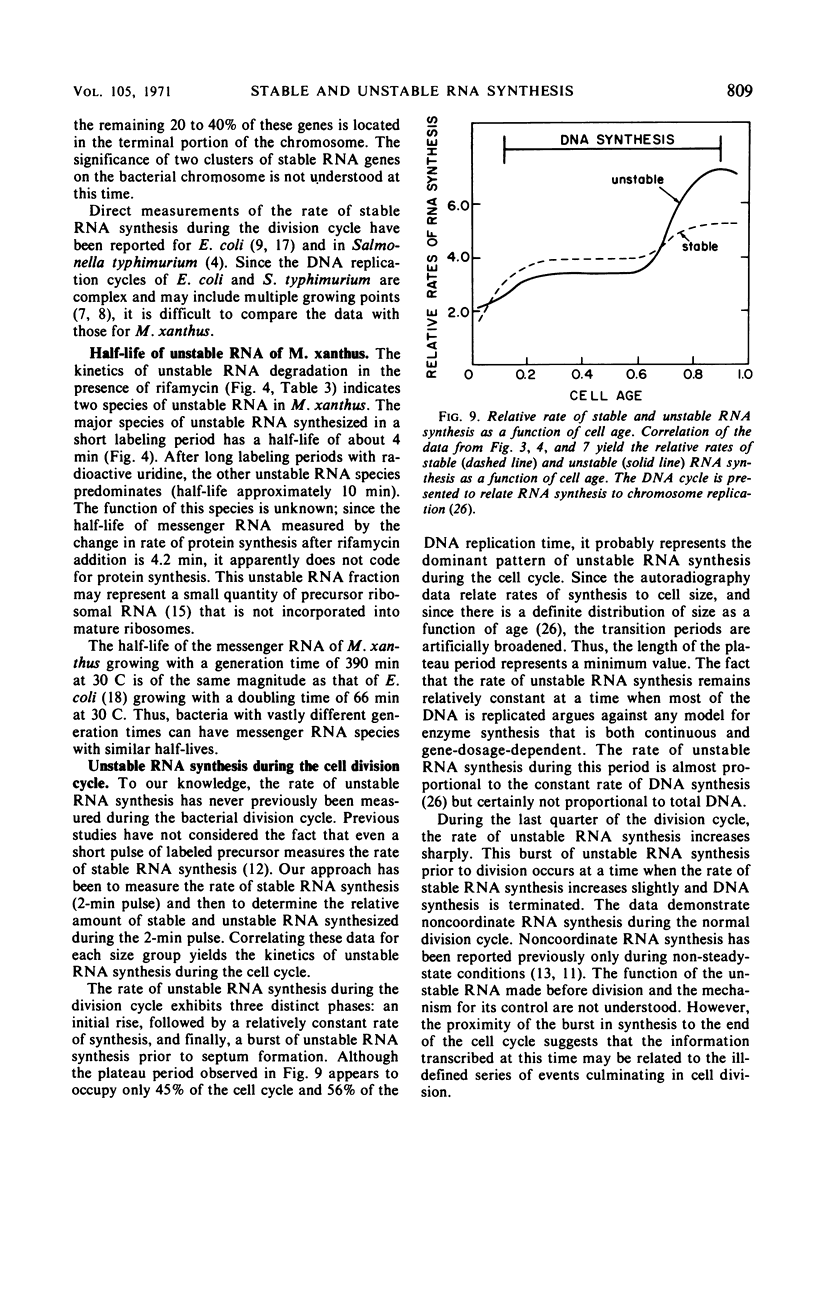
The kinetics of stable and unstable ribonucleic acid (RNA) synthesis during the division cycle of Myxococcus xanthus growing in a defined medium was determined. Under these conditions, M. xanthus contains one chromosome which is replicated during 80% of the cell cycle. Stable RNA synthesis was measured by pulselabeling an exponential-phase culture with radioactive uridine and then preparing the cells for quantitative autoradiography. By measuring the size of individual cells as well as the number of grains, the rate of stable RNA synthesis as a function of cell size was determined. Unstable RNA synthesis during the division cycle was determined by correlating the data for stable RNA synthesis with the relative amounts of stable and unstable RNA labeled during the short pulse. The data reported here demonstrate that: (i) cells synthesize both stable and unstable RNA throughout the division cycle; (ii) the rate of stable RNA synthesis increases in two discrete steps, corresponding to average ages of 0.15 and 0.75 generations; (iii) the rate of unstable RNA synthesis exhibits an initial rise, followed by a relatively constant rate of synthesis, and finally, a burst of unstable RNA synthesis prior to septum formation. The half-life of unstable RNA of M. xanthus, generation time of 390 min at 30 C, was 4 min. Comparison of the rates of stable and unstable RNA synthesis indicates noncoordinate RNA synthesis within the normal division cycle.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colli W., Oishi M. Ribosomal RNA genes in bacteria: evidence for the nature of the physical linkage between 16S and 23S RNA genes in Bacillus subtilis. Proc Natl Acad Sci U S A. 1969 Oct;64(2):642–649. doi: 10.1073/pnas.64.2.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler R. G., Evans J. E. Relative transcription activity of different segments of the genome throughout the cell division cycle of Escherichia coli. The mapping of ribosomal and transfer RNA and the determination of the direction of replication. J Mol Biol. 1967 May 28;26(1):91–105. doi: 10.1016/0022-2836(67)90263-x. [DOI] [PubMed] [Google Scholar]
- Ecker R. E., Kokaisl G. Synthesis of protein, ribonucleic acid, and ribosomes by individual bacterial cells in balanced growth. J Bacteriol. 1969 Jun;98(3):1219–1226. doi: 10.1128/jb.98.3.1219-1226.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANAWALT P. C., MAALOE O., CUMMINGS D. J., SCHAECHTER M. The normal DNA replication cycle. II. J Mol Biol. 1961 Apr;3:156–165. doi: 10.1016/s0022-2836(61)80042-9. [DOI] [PubMed] [Google Scholar]
- Harvey R. J., Marr A. G., Painter P. R. Kinetics of growth of individual cells of Escherichia coli and Azotobacter agilis. J Bacteriol. 1967 Feb;93(2):605–617. doi: 10.1128/jb.93.2.605-617.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lark C. Regulation of deoxyribonucleic acid synthesis in Escherichia coli: dependence on growth rates. Biochim Biophys Acta. 1966 Jun 22;119(3):517–525. doi: 10.1016/0005-2787(66)90128-6. [DOI] [PubMed] [Google Scholar]
- Manor H., Haselkorn R. Size fractionation of exponentially growing Escherichia coli. Nature. 1967 Jun 3;214(5092):983–986. doi: 10.1038/214983a0. [DOI] [PubMed] [Google Scholar]
- Mitchison J. M. Enzyme synthesis in synchronous cultures. Science. 1969 Aug 15;165(3894):657–663. doi: 10.1126/science.165.3894.657. [DOI] [PubMed] [Google Scholar]
- Morris D. W., Kjeldgaard N. O. Evidence for the non-co-ordinate regulation of ribonucleic acid synthesis in stringent strains of Escherichia coli. J Mol Biol. 1968 Jan 14;31(1):145–148. doi: 10.1016/0022-2836(68)90064-8. [DOI] [PubMed] [Google Scholar]
- Nierlich D. P. Amino acid control over RNA synthesis: a re-evaluation. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1345–1352. doi: 10.1073/pnas.60.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierlich D. P. Radioisotope uptake as a measure of synthesis of messenger RNA. Science. 1967 Dec 1;158(3805):1186–1188. doi: 10.1126/science.158.3805.1186. [DOI] [PubMed] [Google Scholar]
- Nierlich D. P., Vielmetter W. Kinetic studies on the relationship of ribonucleotide precursor pools and ribonucleic acid synthesis. J Mol Biol. 1968 Feb 28;32(1):135–147. doi: 10.1016/0022-2836(68)90151-4. [DOI] [PubMed] [Google Scholar]
- Pace B., Peterson R. L., Pace N. R. Formation of all stable RNA species in Escherichia coli by posttranscriptional modification. Proc Natl Acad Sci U S A. 1970 Apr;65(4):1097–1104. doi: 10.1073/pnas.65.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci O., Helmstetter C. E. Chromosome replication, protein synthesis and cell division in Escherichia coli. Fed Proc. 1969 Nov-Dec;28(6):1755–1760. [PubMed] [Google Scholar]
- Rudner R., Rejman E., Chargaff E. Genetic implications of periodic pulsations of the rate of synthesis and the composition of rapidly labeled bacterial RNA. Proc Natl Acad Sci U S A. 1965 Sep;54(3):904–911. doi: 10.1073/pnas.54.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salser W., Janin J., Levinthal C. Measurement of the unstable RNA in exponentially growing cultures of Bacillus subtilis and Escherichia coli. J Mol Biol. 1968 Jan 28;31(2):237–266. doi: 10.1016/0022-2836(68)90442-7. [DOI] [PubMed] [Google Scholar]
- Sippel A., Hartmann G. Mode of action of rafamycin on the RNA polymerase reaction. Biochim Biophys Acta. 1968 Mar 18;157(1):218–219. doi: 10.1016/0005-2787(68)90286-4. [DOI] [PubMed] [Google Scholar]
- Smith I., Dubnau D., Morrell P., Marmur J. Chromosomal location of DNA base sequences complementary to transfer RNA and to 5 s, 16 s and 23 s ribosomal RNA in Bacillus subtilis. J Mol Biol. 1968 Apr 14;33(1):123–140. doi: 10.1016/0022-2836(68)90285-4. [DOI] [PubMed] [Google Scholar]
- Stanley W. M., Jr, Bock R. M. Isolation and physical properties of the ribosomal ribonucleic acid of Escherichia coli. Biochemistry. 1965 Jul;4(7):1302–1311. doi: 10.1021/bi00883a014. [DOI] [PubMed] [Google Scholar]
- Wehrli W., Nüesch J., Knüsel F., Staehelin M. Action of rifamycins on RNA polymerase. Biochim Biophys Acta. 1968 Mar 18;157(1):215–217. doi: 10.1016/0005-2787(68)90285-2. [DOI] [PubMed] [Google Scholar]
- Witkin S. S., Rosenberg E. Induction of morphogenesis by methionine starvation in Myxococcus xanthus: polyamine control. J Bacteriol. 1970 Sep;103(3):641–649. doi: 10.1128/jb.103.3.641-649.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zusman D., Gottlieb P., Rosenberg E. Division cycle of Myxococcus xanthus. 3. Kinetics of cell growth and protein synthesis. J Bacteriol. 1971 Mar;105(3):811–819. doi: 10.1128/jb.105.3.811-819.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zusman D., Rosenberg E. DNA cycle of Myxococcus xanthus. J Mol Biol. 1970 May 14;49(3):609–619. doi: 10.1016/0022-2836(70)90285-8. [DOI] [PubMed] [Google Scholar]
- Zusman D., Rosenberg E. Deoxyribonucleic acid synthesis during microcyst germination in Myxococcus xanthus. J Bacteriol. 1968 Oct;96(4):981–986. doi: 10.1128/jb.96.4.981-986.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


