Abstract
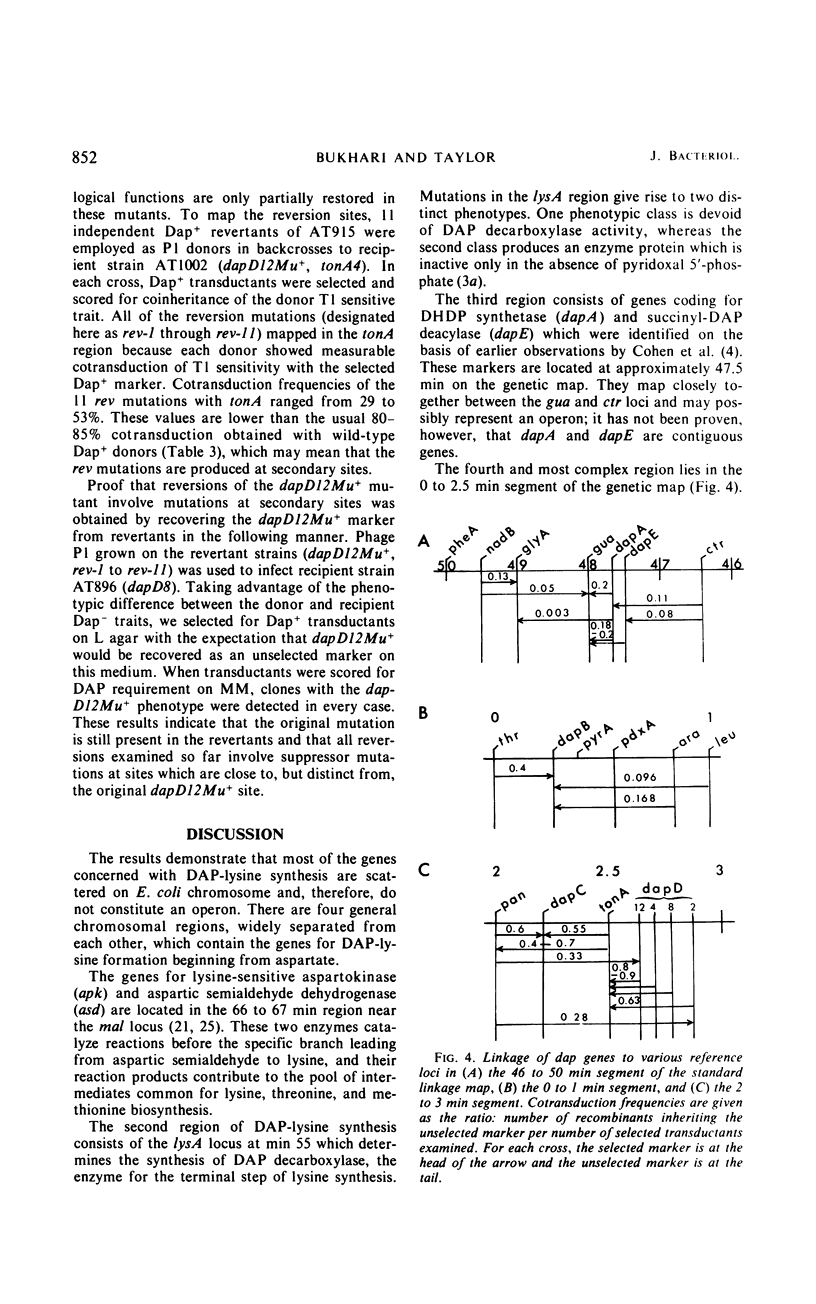
Several diaminopimelic acid (DAP)- and lysine-requiring mutants of Escherichia coli were isolated and studied by genetic, physiological, and biochemical means. The genes concerned with DAP-lysine synthesis map at several different sites on the E. coli chromosome and, therefore, do not constitute a single operon. Three separate loci affecting DAP synthesis are located in the 0 to 2.5 min region of the genetic map. The order of the loci in this region is thr-dapB-pyrA-ara-leu-pan-dapC-tonA-dapD. Two additional DAP genes map in the region between min 47 and 48, with the gene order being gua-dapA-dapE-ctr. The lys locus at min 55 determines the synthesis of the enzyme DAP decarboxylase, which catalyzes the conversion of DAP into lysine. The order of the genes in this region is serA-lysA-thyA.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTIA M., HOARE D. S., WORK E. The stereoisomers of alpha epsilon-diaminopimelic acid. III. Properties and distribution of diaminopimelic acid racemase, an enzyme causing interconversion of the LL and meso isomers. Biochem J. 1957 Mar;65(3):448–459. doi: 10.1042/bj0650448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes I. J., Bondi A., Moat A. G. Biochemical characterization of lysine auxotrophs of Staphylococcus aureus. J Bacteriol. 1969 Jul;99(1):169–174. doi: 10.1128/jb.99.1.169-174.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari A. I., Taylor A. L. Mutants of Escherichia coli with a growth requirement for either lysine or pyridoxine. J Bacteriol. 1971 Mar;105(3):988–998. doi: 10.1128/jb.105.3.988-998.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas W., Gilvarg C. The reduction step in diaminopimelic acid biosynthesis. J Biol Chem. 1965 Dec;240(12):4717–4722. [PubMed] [Google Scholar]
- Fukuda A., Gilvarg C. The relationship of dipicolinate and lysine biosynthesis in Bacillus megaterium. J Biol Chem. 1968 Jul 25;243(14):3871–3876. [PubMed] [Google Scholar]
- GILVARG C. N-Succinyl-L-diaminopimelic acid. J Biol Chem. 1959 Nov;234:2955–2959. [PubMed] [Google Scholar]
- GILVARG C. N-Succinyl-alpha-amino-6-ketopimelic acid. J Biol Chem. 1961 May;236:1429–1431. [PubMed] [Google Scholar]
- GILVARG C. The branching point in diaminopimelic acid synthesis. J Biol Chem. 1962 Feb;237:482–484. [PubMed] [Google Scholar]
- GILVARG C. The enzymatic synthesis of diaminopimelic acid. J Biol Chem. 1958 Dec;233(6):1501–1504. [PubMed] [Google Scholar]
- HOARE D. S., WORK E. The stereoisomers of alpha epsilon-diaminopimelic acid: their distribution in nature and behaviour towards certain enzyme preparations. Biochem J. 1955 Dec;61(4):562–568. doi: 10.1042/bj0610562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINDLER S. H., GILVARG C. N-Succinyl-L-2,6-diaminopimelic acid deacylase. J Biol Chem. 1960 Dec;235:3532–3535. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin H. H. Biochemistry of bacterial cell walls. Annu Rev Biochem. 1966;35:457–484. doi: 10.1146/annurev.bi.35.070166.002325. [DOI] [PubMed] [Google Scholar]
- Matsuzawa H., Matsuhashi M., Oka A., Sugino Y. Genetic and biochemical studies on cell wall peptidoglycan synthesis in Escherichia coli K-12. Biochem Biophys Res Commun. 1969 Aug 15;36(4):682–689. doi: 10.1016/0006-291x(69)90360-x. [DOI] [PubMed] [Google Scholar]
- Normark S., Boman H. G., Matsson E. Mutant of Escherichia coli with anomalous cell division and ability to decrease episomally and chromosomally mediated resistance to ampicillin and several other antibiotics. J Bacteriol. 1969 Mar;97(3):1334–1342. doi: 10.1128/jb.97.3.1334-1342.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERKOFSKY B., GILVARG C. N-Succinyl-L-diaminopimelic-glutamic transaminase. J Biol Chem. 1961 May;236:1432–1438. [PubMed] [Google Scholar]
- Patte J. C., Cohen G. N. Isolement et propriétés d'un mutant d'Escherichia coli depourvu d'aspartokinase sensible à la lysine. Biochim Biophys Acta. 1965 Jun 22;99(3):561–563. [PubMed] [Google Scholar]
- SALTON M. R. Studies of the bacterial cell wall. IV. The composition of the cell walls of some Gram-positive and Gram-negative bacteria. Biochim Biophys Acta. 1953 Apr;10(4):512–523. doi: 10.1016/0006-3002(53)90296-0. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Location of the maltose A and B loci on the genetic map of Escherichia coli. J Bacteriol. 1966 Oct;92(4):1083–1089. doi: 10.1128/jb.92.4.1083-1089.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman E. R. Allosteric regulation of enzyme activity. Adv Enzymol Relat Areas Mol Biol. 1966;28:41–154. doi: 10.1002/9780470122730.ch2. [DOI] [PubMed] [Google Scholar]
- TAYLOR A. L. BACTERIOPHAGE-INDUCED MUTATION IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1963 Dec;50:1043–1051. doi: 10.1073/pnas.50.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritz G. J., Matney T. S., Gholson R. K. Mapping of the nadB locus adjacent to a previously undescribed purine locus in Escherichia coli K-12. J Bacteriol. 1970 May;102(2):377–381. doi: 10.1128/jb.102.2.377-381.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truffa-Bachi P., Cohen G. N. Some aspects of amino acid biosynthesis in microorganisms. Annu Rev Biochem. 1968;37:79–108. doi: 10.1146/annurev.bi.37.070168.000455. [DOI] [PubMed] [Google Scholar]
- WHITE P. J., KELLY B. PURIFICATION AND PROPERTIES OF DIAMINOPIMELATE DECARBOXYLASE FROM ESCHERICHIA COLI. Biochem J. 1965 Jul;96:75–84. doi: 10.1042/bj0960075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WORK E. A new naturally occurring amino acid. Nature. 1950 Jan 14;165(4185):74–74. doi: 10.1038/165074b0. [DOI] [PubMed] [Google Scholar]
- Wang R. J., Morse H. G., Morse M. L. Carbohydrate accumulation and metabolism in Escherichia coli: the close linkage and chromosomal location of ctr mutations. J Bacteriol. 1969 May;98(2):605–610. doi: 10.1128/jb.98.2.605-610.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. J., Kelly B., Suffling A., Work E. Variation of activity of bacterial diaminopimelate decarboxylase under different conditions of growth. Biochem J. 1964 Jun;91(3):600–610. doi: 10.1042/bj0910600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yugari Y., Gilvarg C. The condensation step in diaminopimelate synthesis. J Biol Chem. 1965 Dec;240(12):4710–4716. [PubMed] [Google Scholar]


