Abstract
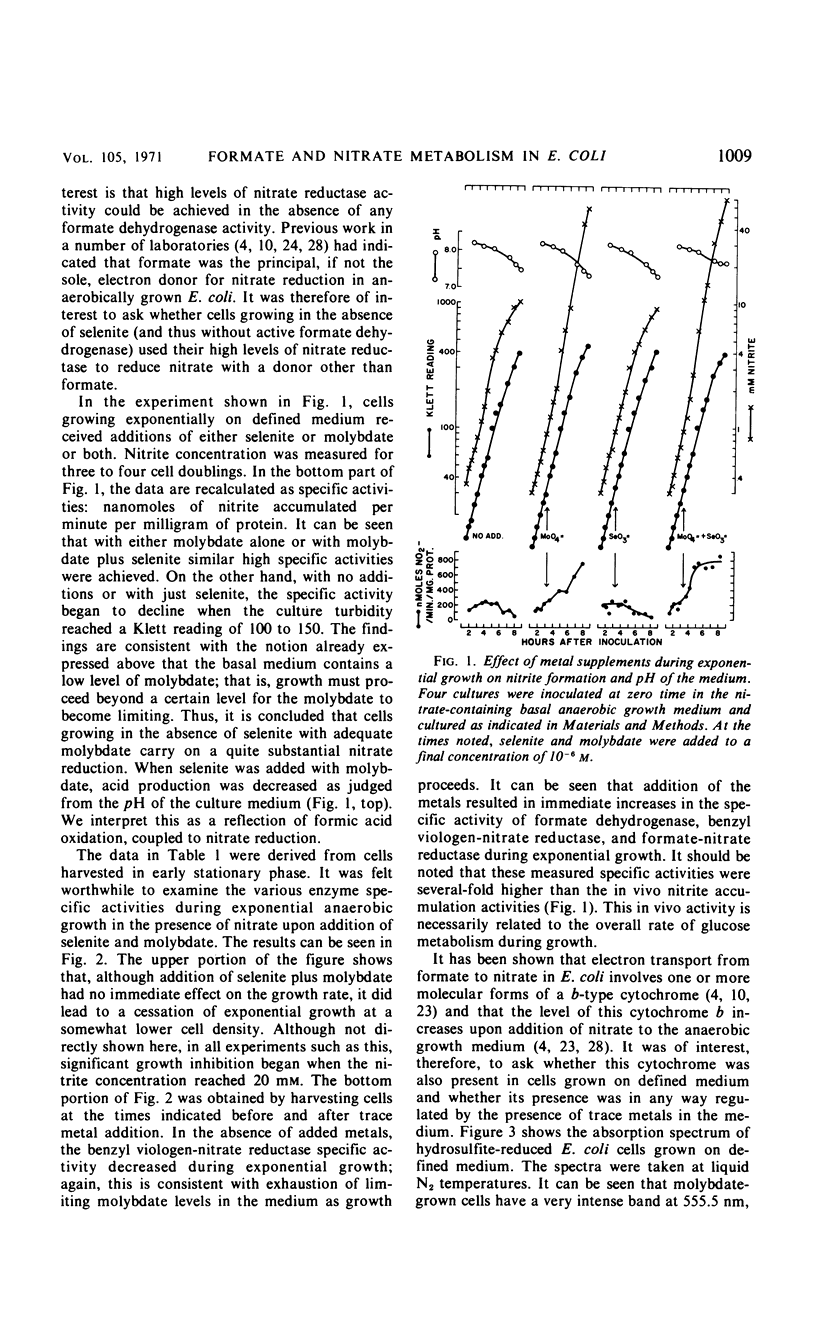
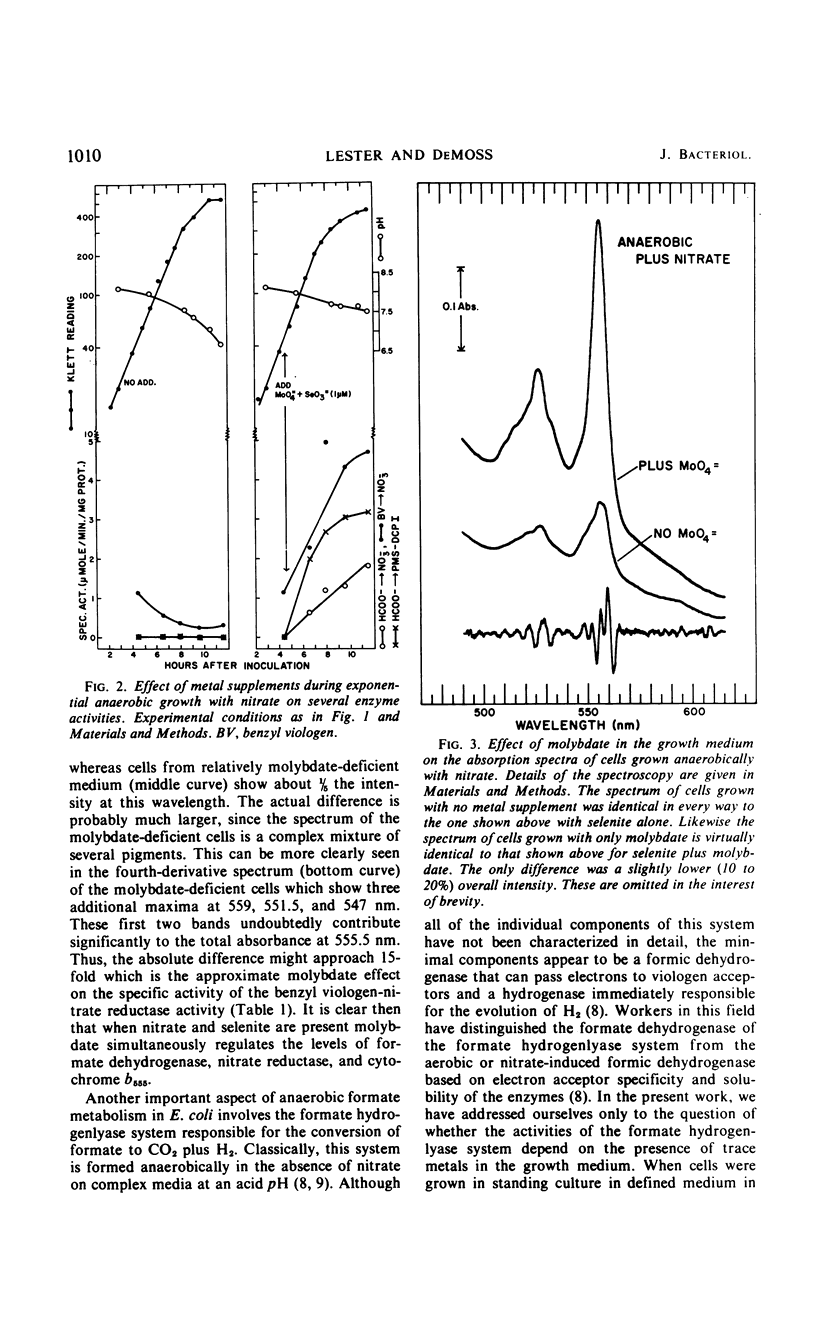
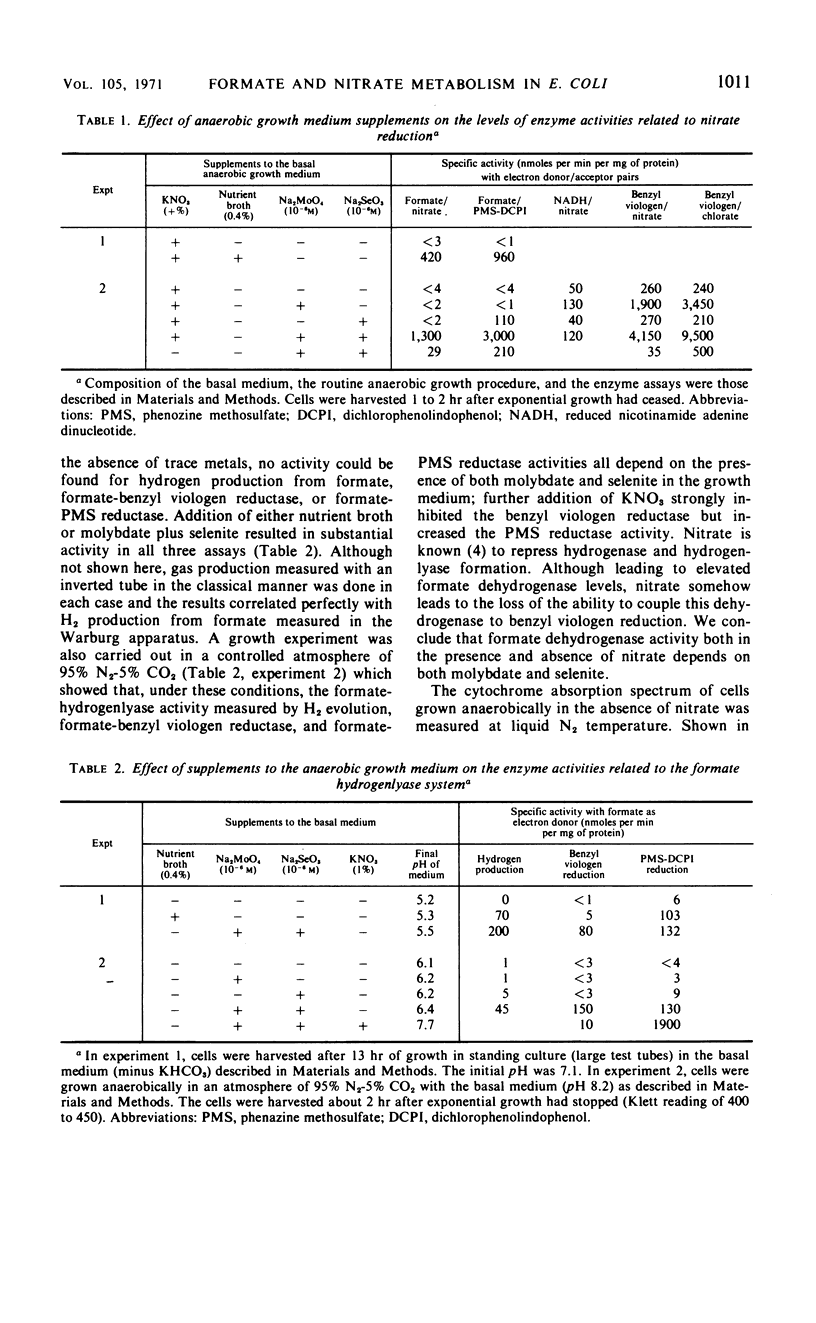
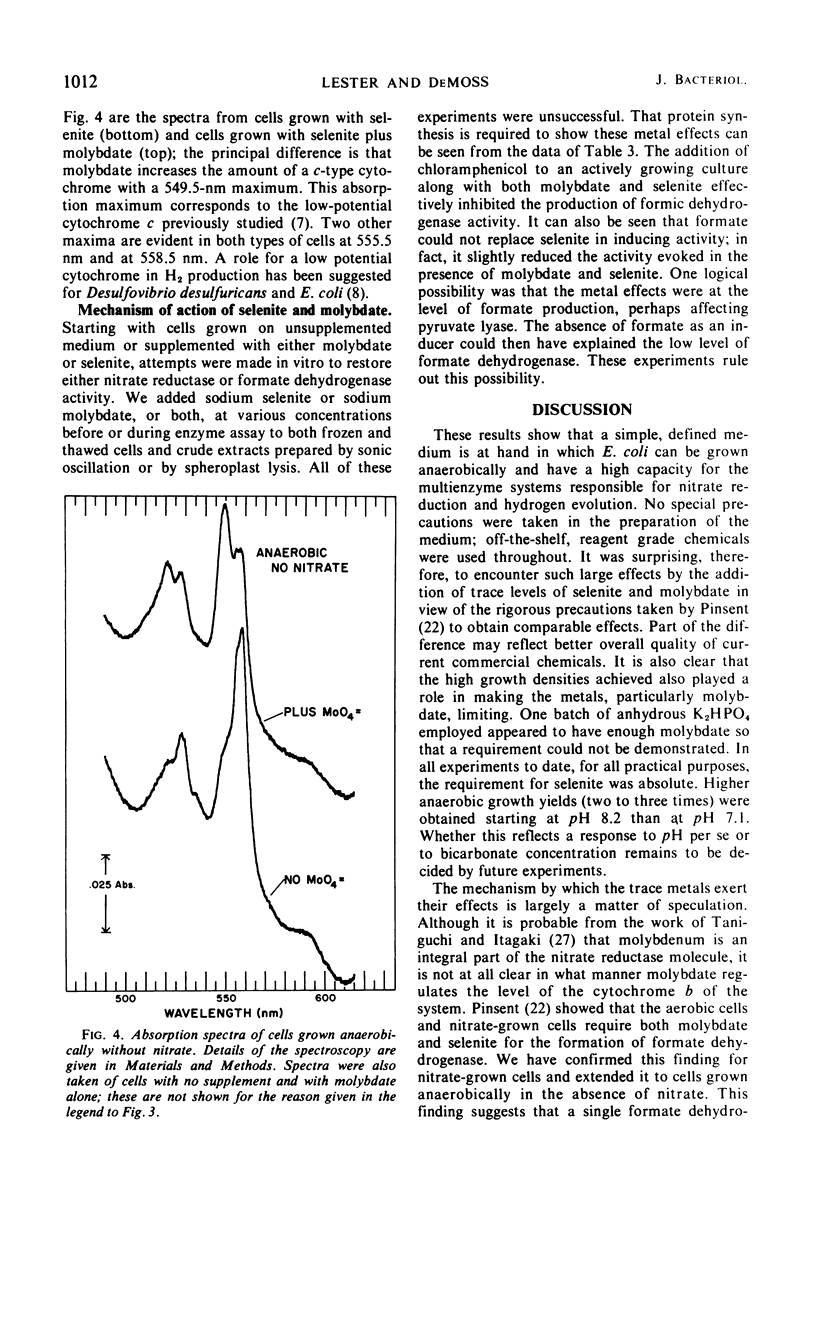
The effects of adding molybdate and selenite to a glucose-minimal salts medium on the formation of enzymes involved in the anaerobic metabolism of formate and nitrate in Escherichia coli have been studied. When cells were grown anaerobically in the presence of nitrate, molybdate stimulated the formation of nitrate reductase and a b-type cytochrome, resulting in cells that had the capacity for active nitrate reduction in the absence of formate dehydrogenase. Under the same conditions, selenite in addition to molybdate was required for forming the enzyme system which permits formate to serve as an effective electron donor for nitrate reduction. When cells were grown anaerobically on a glucose-minimal salts medium without nitrate, active hydrogen production from formate as well as formate dehydrogenase activity depended on the presence of both selenite and molybdate. The effects of these metals on the formation of formate dehydrogenase was blocked by chloramphenicol, suggesting that protein synthesis is required for the increases observed. It is proposed that the same formate dehydrogenase is involved in nitrate reduction, hydrogen production, and in aerobic formate oxidation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azoulay E., Puig J., Couchoud-Beaumont P. Etude des mutants chlorate-résistants chez Escherichia coli K 12. I. Reconstitution in vitro de l'activité nitrate-réductase particulaire chez Escherichia coli K 12. Biochim Biophys Acta. 1969 Feb 11;171(2):238–252. doi: 10.1016/0005-2744(69)90157-0. [DOI] [PubMed] [Google Scholar]
- Cole J. A., Wimpenny J. W. Metabolic pathways for nitrate reduction in Escherichia coli. Biochim Biophys Acta. 1968 Jul 16;162(1):39–48. doi: 10.1016/0005-2728(68)90212-0. [DOI] [PubMed] [Google Scholar]
- Fukuyama T., Ordal E. J. Induced Biosynthesis of Formic Hydrogenlyase in Iron-Deficient Cells of Escherichia coli. J Bacteriol. 1965 Sep;90(3):673–680. doi: 10.1128/jb.90.3.673-680.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEST H., PECK H. D., Jr A study of the hydrogenlyase reaction with systems derived from normal and anaerogenic coli-aerogenes bacteria. J Bacteriol. 1955 Sep;70(3):326–334. doi: 10.1128/jb.70.3.326-334.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY C. T., GEST H. BIOLOGICAL FORMATION OF MOLECULAR HYDROGEN. Science. 1965 Apr 9;148(3667):186–192. doi: 10.1126/science.148.3667.186. [DOI] [PubMed] [Google Scholar]
- GRAY C. T., WIMPENNY J. W., HUGHES D. E., RANLETT M. A soluble c-type cytochrome from anaerobically grown Escherichia coli and various Enterobacteriaceae. Biochim Biophys Acta. 1963 Jan 8;67:157–160. doi: 10.1016/0006-3002(63)91809-2. [DOI] [PubMed] [Google Scholar]
- Gray C. T., Wimpenny J. W., Hughes D. E., Mossman M. R. Regulation of metabolism in facultative bacteria. I. Structural and functional changes in Escherichia coli associated with shifts between the aerobic and anaerobic states. Biochim Biophys Acta. 1966 Mar 28;117(1):22–32. doi: 10.1016/0304-4165(66)90148-6. [DOI] [PubMed] [Google Scholar]
- LINNANE A. W., WRIGLEY C. W. FRAGMENTATION OF THE ELECTRON TRANSPORT CHAIN OF ESCHERICHIA COLI. PREPARATION OF A SOLUBLE FORMATE DEHYDROGENASE-CYTOCHROME B1 COMPLEX. Biochim Biophys Acta. 1963 Nov 8;77:408–418. doi: 10.1016/0006-3002(63)90515-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- O'Hara J., Gray C. T. Defects in formate hydrogenlyase in nitrate-negative mutants of Escherichia coli. Biochem Biophys Res Commun. 1967 Sep 27;28(6):951–957. doi: 10.1016/0006-291x(67)90072-1. [DOI] [PubMed] [Google Scholar]
- PECK H. D., Jr, GEST H. Formic dehydrogenase and the hydrogenlyase enzyme complex in coli-aerogenes bacteria. J Bacteriol. 1957 Jun;73(6):706–721. doi: 10.1128/jb.73.6.706-721.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PICHINOTY F. [Inhibition by oxygen of the biosynthesis and activity of hydrogenase and hydrogenlyase in some anaerobic bacteria]. Biochim Biophys Acta. 1962 Oct 8;64:111–124. doi: 10.1016/0006-3002(62)90764-3. [DOI] [PubMed] [Google Scholar]
- PICHINOTY F., d' ORNANO [Influence of the culture conditions on the formation of nitrate reductase of Aerobacter aerogenes]. Biochim Biophys Acta. 1961 Mar 18;48:218–220. doi: 10.1016/0006-3002(61)90783-1. [DOI] [PubMed] [Google Scholar]
- PINSENT J. The need for selenite and molybdate in the formation of formic dehydrogenase by members of the coli-aerogenes group of bacteria. Biochem J. 1954 May;57(1):10–16. doi: 10.1042/bj0570010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichinoty F. Les nitrate-réductases bactériennes. II. Comportement de l'enzyme A envers les donneurs d'électrons. Arch Mikrobiol. 1969;68(1):65–73. [PubMed] [Google Scholar]
- Pichinoty F., Puig J., Chippaux M., Bigliardi-Rouvier J., Gendre J. Recherches sur des mutants bactériens ayant perdu les activités catalytiques liées à la nitrate-réductase A. II. Comportement envers le chlorate et le chlorite. Ann Inst Pasteur (Paris) 1969 Apr;116(4):409–432. [PubMed] [Google Scholar]
- Pichinoty F. Recherche des activités formiate-oxydase, hydrogène-lyase, hydrogénase et formiate-déshydrogénase chez quelques enterobacteriaceae. Ann Inst Pasteur (Paris) 1969 Jul;117(1):3–15. [PubMed] [Google Scholar]
- Piéchaud M., Puig J., Pichinoty F., Azoulay E., Le Minor L. Mutations affectant la nitrate-réductase A et d'autres enzymes bactériennes d'oxydoréduction. Ann Inst Pasteur (Paris) 1967 Jan;112(1):24–37. [PubMed] [Google Scholar]
- Ruiz-Herrera J., DeMoss J. A. Nitrate reductase complex of Escherichia coli K-12: participation of specific formate dehydrogenase and cytochrome b1 components in nitrate reduction. J Bacteriol. 1969 Sep;99(3):720–729. doi: 10.1128/jb.99.3.720-729.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Herrera J., Showe M. K., DeMoss J. A. Nitrate reductase complex of Escherichia coli K-12: isolation and characterization of mutants unable to reduce nitrate. J Bacteriol. 1969 Mar;97(3):1291–1297. doi: 10.1128/jb.97.3.1291-1297.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showe M. K., DeMoss J. A. Localization and regulation of synthesis of nitrate reductase in Escherichia coli. J Bacteriol. 1968 Apr;95(4):1305–1313. doi: 10.1128/jb.95.4.1305-1313.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouthamer A. H. Nitrate reduction in Aerobacter aerogenes. II. Characterization of mutants blocked in the reduction of nitrate and chlorate. Arch Mikrobiol. 1967 Feb 1;56(1):76–80. [PubMed] [Google Scholar]
- TANIGUCHI S., ITAGAKI E. Nitrate reductase of nitrate respiration type from E. coli. I. Solubilization and purification from the particulate system with molecular characterization as a metalloprotein. Biochim Biophys Acta. 1960 Nov 4;44:263–279. doi: 10.1016/0006-3002(60)91562-6. [DOI] [PubMed] [Google Scholar]
- Wimpenny J. W., Cole J. A. The regulation of metabolism in facultative bacteria. 3. The effect of nitrate. Biochim Biophys Acta. 1967 Oct 9;148(1):233–242. doi: 10.1016/0304-4165(67)90298-x. [DOI] [PubMed] [Google Scholar]
- Yu L., Wolin M. J. Hydrogenase measurement with photochemically reduced methyl viologen. J Bacteriol. 1969 Apr;98(1):51–55. doi: 10.1128/jb.98.1.51-55.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


