Abstract
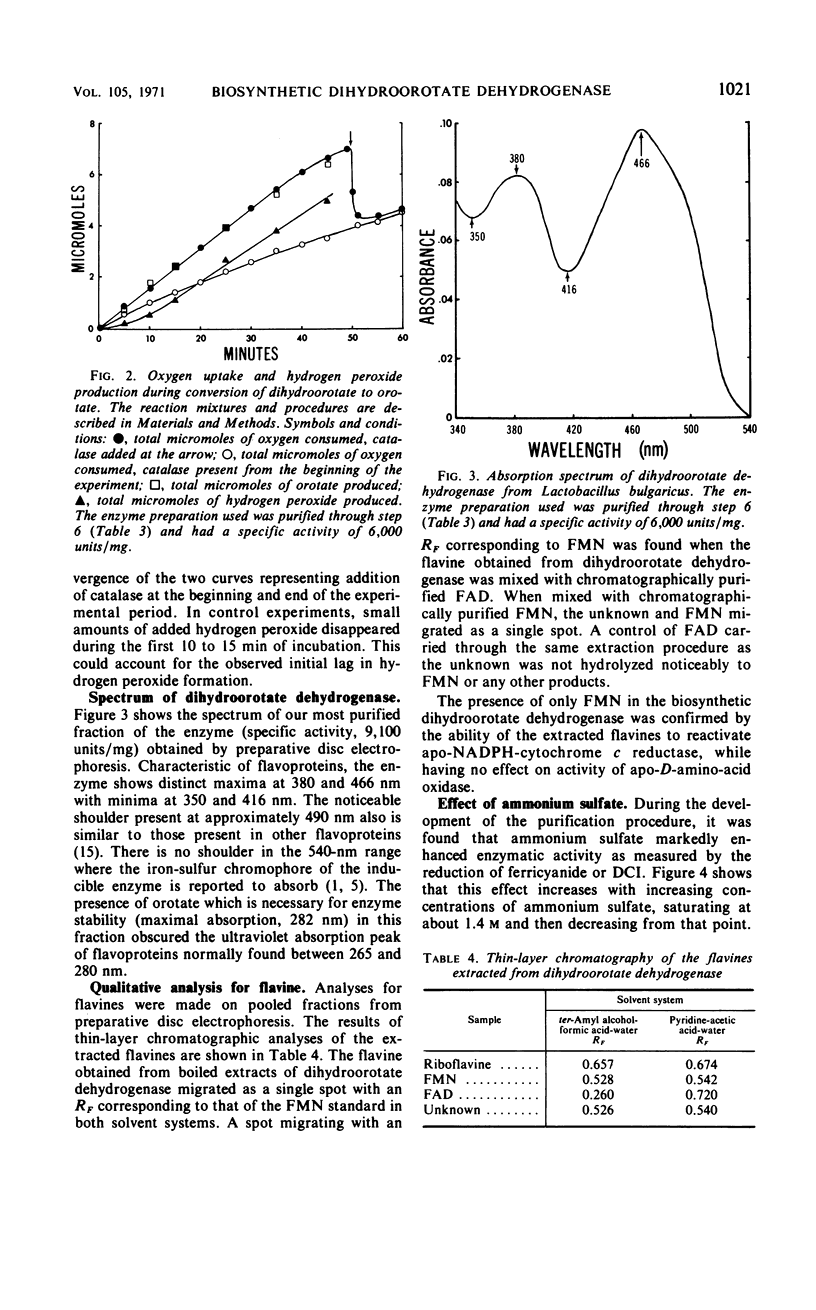
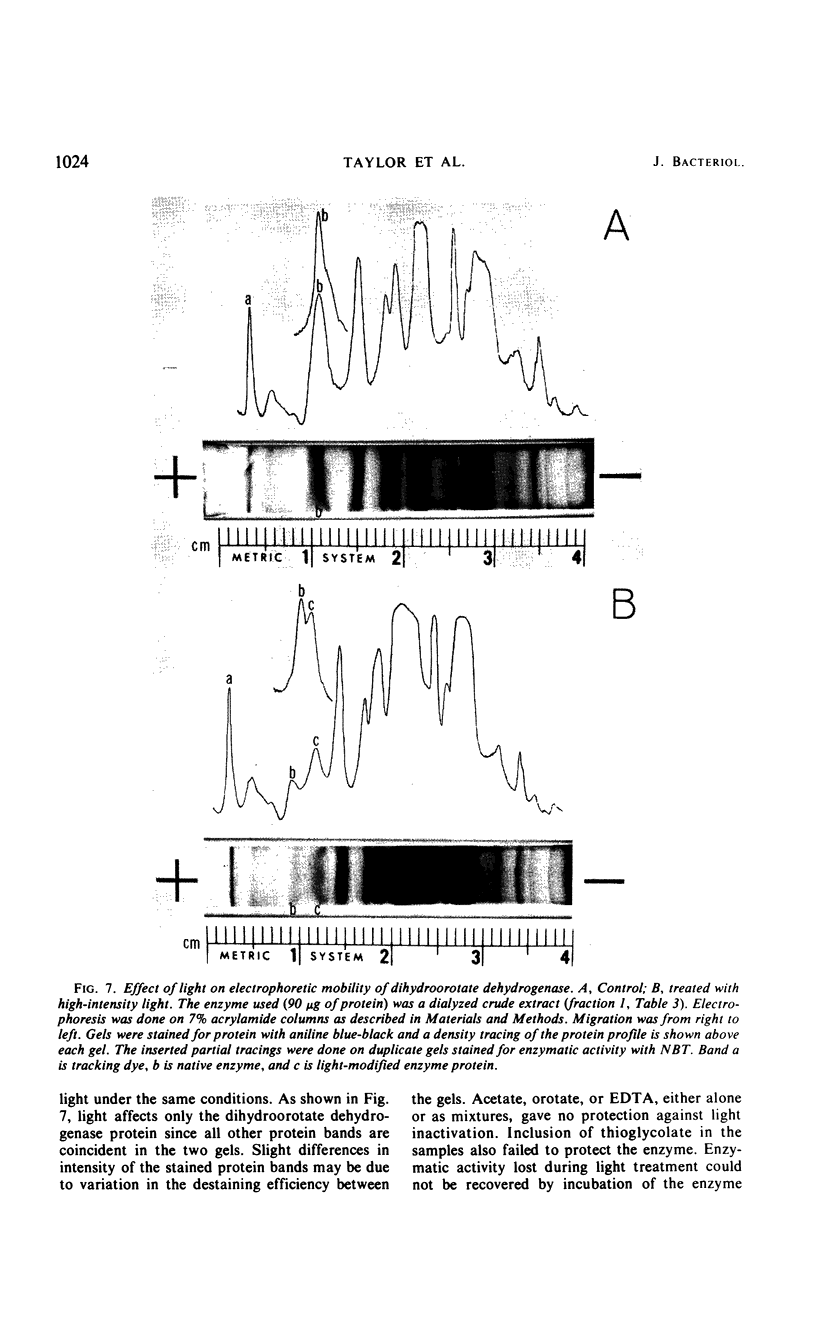
This paper describes the first detailed study on a dihydroorotate dehydrogenase involved in pyrimidine biosynthesis. In most organisms the enzyme is membrane-bound; however, a soluble dihydroorotate dehydrogenase was produced in relatively high levels when the anaerobe, Lactobacillus bulgaricus, was released from repression. The enzyme was purified 213-fold over derepressed levels with a 39% recovery of enzyme units. The enzyme showed only one minor protein contaminant when analyzed by polyacrylamide electrophoresis. It was characterized as a flavoprotein containing only flavine mononucleotide as the prosthetic group. Molecular weight estimations by gel filtration gave a value of approximately 55,000, which is one-half that of the degradative enzyme described by others. During aerobic oxidation of dihydroorotate, the rates of oxygen consumption, orotate formation, and hydrogen peroxide formation were equal, as would be expected in a flavoprotein-catalyzed reaction. The enzymatic activity with ferricyanide as acceptor was optimum around pH 7.7. The stimulation of enzymatic activity over a wide pH range by ammonium sulfate was attributed to an effect on the maximum velocity of the reaction. As analyzed by polyacrylamide electrophoresis, inactivation of the enzyme by visible light resulted in the appearance of a second protein band with lowered specific activity. The purified enzyme used redox dyes, oxygen, or cytochrome c as electron acceptors but was not active with pyridine nucleotides. Flavine adenine dinucleotide has been implicated at the active site for pyridine nucleotide reduction in the degradative enzyme. The biosynthetic enzyme lacks this flavine and the associated activity.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleman V., Handler P. Dihydroorotate dehydrogenase. I. General properties. J Biol Chem. 1967 Sep 25;242(18):4087–4096. [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- FRIEDMANN H. C., VENNESLAND B. Crystalline dihydroorotic dehydrogenase. J Biol Chem. 1960 May;235:1526–1532. [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- JOVIN T., CHRAMBACH A., NAUGHTON M. A. AN APPARATUS FOR PREPARATIVE TEMPERATURE-REGULATED POLYACRYLAMIDE GEL ELECTROPHORESIS. Anal Biochem. 1964 Nov;9:351–369. doi: 10.1016/0003-2697(64)90192-7. [DOI] [PubMed] [Google Scholar]
- KONDO H., FRIEDMANN H. C., VENNESLAND B. Flavin changes accompanying adaptation of Zymobacterium oroticum to orotate. J Biol Chem. 1960 May;235:1533–1535. [PubMed] [Google Scholar]
- Kerr C. T., Miller R. W. Dihydroorotate-ubiquinone reductase complex of Escherichia coli B. J Biol Chem. 1968 Jun 10;243(11):2963–2968. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER R. W., MASSEY V. DIHYDROOROTIC DEHYDROGENASE. I. SOME PROPERTIES OF THE ENZYME. J Biol Chem. 1965 Mar;240:1453–1465. [PubMed] [Google Scholar]
- Miller R. W., Kerr C. T. Dihydroorotate dehydrogenase. 3. Interactions with substrates, inhibitors, artificial electron acceptors, and cytochrome c. J Biol Chem. 1966 Dec 10;241(23):5597–5604. [PubMed] [Google Scholar]
- Miller R. W., Kerr C. T. Particulate dihydroorotate oxidase system from a pseudomonad. Linkage with the respiratory chain. Can J Biochem. 1967 Sep;45(9):1283–1294. doi: 10.1139/o67-150. [DOI] [PubMed] [Google Scholar]
- Nelson C. A., Handler P. Preparation of bovine xanthine oxidase and the subunit structures of some iron flavoproteins. J Biol Chem. 1968 Oct 25;243(20):5368–5373. [PubMed] [Google Scholar]
- TAYLOR W. H., NOVELLI G. D. ENZYMES OF THE PYRIMIDINE PATHWAY IN ESCHERICHIA COLI. I. SYNTHESIS BY CELLS AND SPHEROPLASTS. J Bacteriol. 1964 Jul;88:99–104. doi: 10.1128/jb.88.1.99-104.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR W. H., TAYLOR M. L. ENZYMES OF THE PYRIMIDINE PATHWAY IN ESCHERICHIA COLI. II. INTRACELLULAR LOCALIZATION AND PROPERTIES OF DIHYDROOROTIC DEHYDROGENASE. J Bacteriol. 1964 Jul;88:105–110. doi: 10.1128/jb.88.1.105-110.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W. H., Taylor M. L., Eames D. F. Two functionally different dihydroorotic dehydrogenases in bacteria. J Bacteriol. 1966 Jun;91(6):2251–2256. doi: 10.1128/jb.91.6.2251-2256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UDAKA S., VENNESLAND B. Properties of triphosphopyridine nucleotide-linked dihydroorotic dehydrogenase. J Biol Chem. 1962 Jun;237:2018–2024. [PubMed] [Google Scholar]
- WEBB E. C., MORROW P. F. The activation of an arysulphatase from ox liver by chloride and other anions. Biochem J. 1959 Sep;73:7–15. doi: 10.1042/bj0730007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WRIGHT L. D., DRISCOLL C. A., MILLER C. S., SKEGGS H. R. Dihydroorotic acid in nutrition of lactic acid bacteria. Proc Soc Exp Biol Med. 1953 Dec;84(3):716–719. doi: 10.3181/00379727-84-20763. [DOI] [PubMed] [Google Scholar]
- WRIGHT L. D., VALENTIK K. A., SPICER D. S., HUFF J. W., SKEGGS H. R. Orotic acid and related compounds in the nutrition of Lactobacillus bulgaricus 09. Proc Soc Exp Biol Med. 1950 Oct;75(1):293–297. doi: 10.3181/00379727-75-18175. [DOI] [PubMed] [Google Scholar]
- YATES R. A., PARDEE A. B. Control by uracil of formation of enzymes required for orotate synthesis. J Biol Chem. 1957 Aug;227(2):677–692. [PubMed] [Google Scholar]




