Abstract
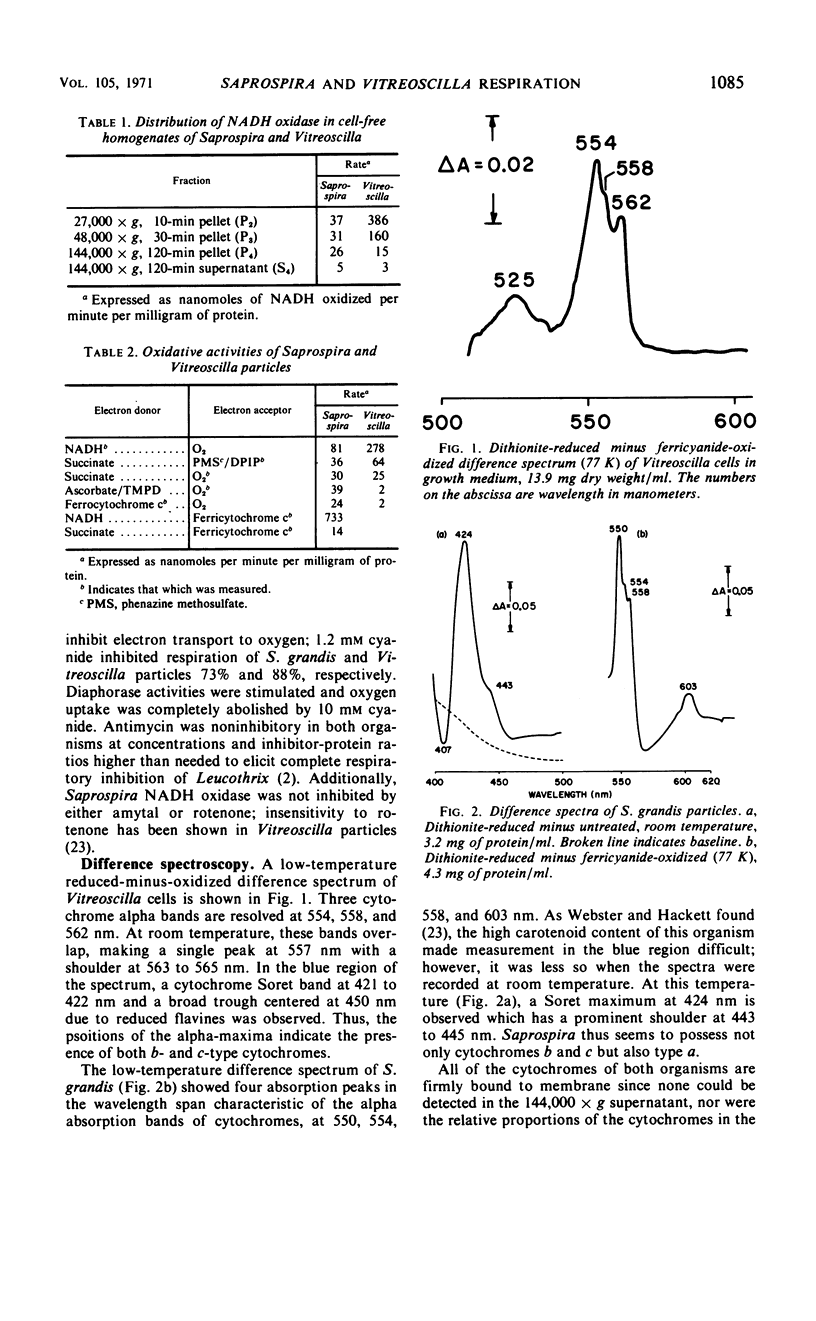
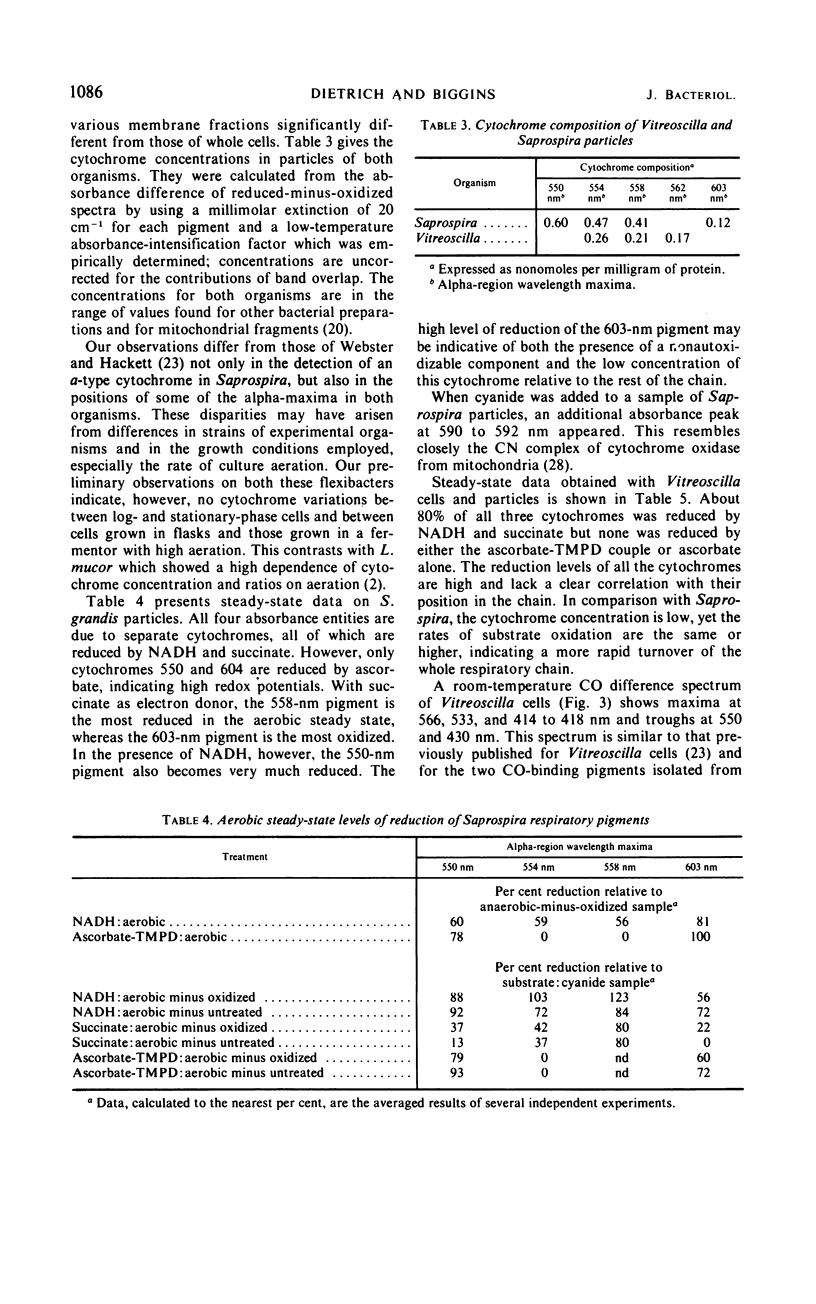
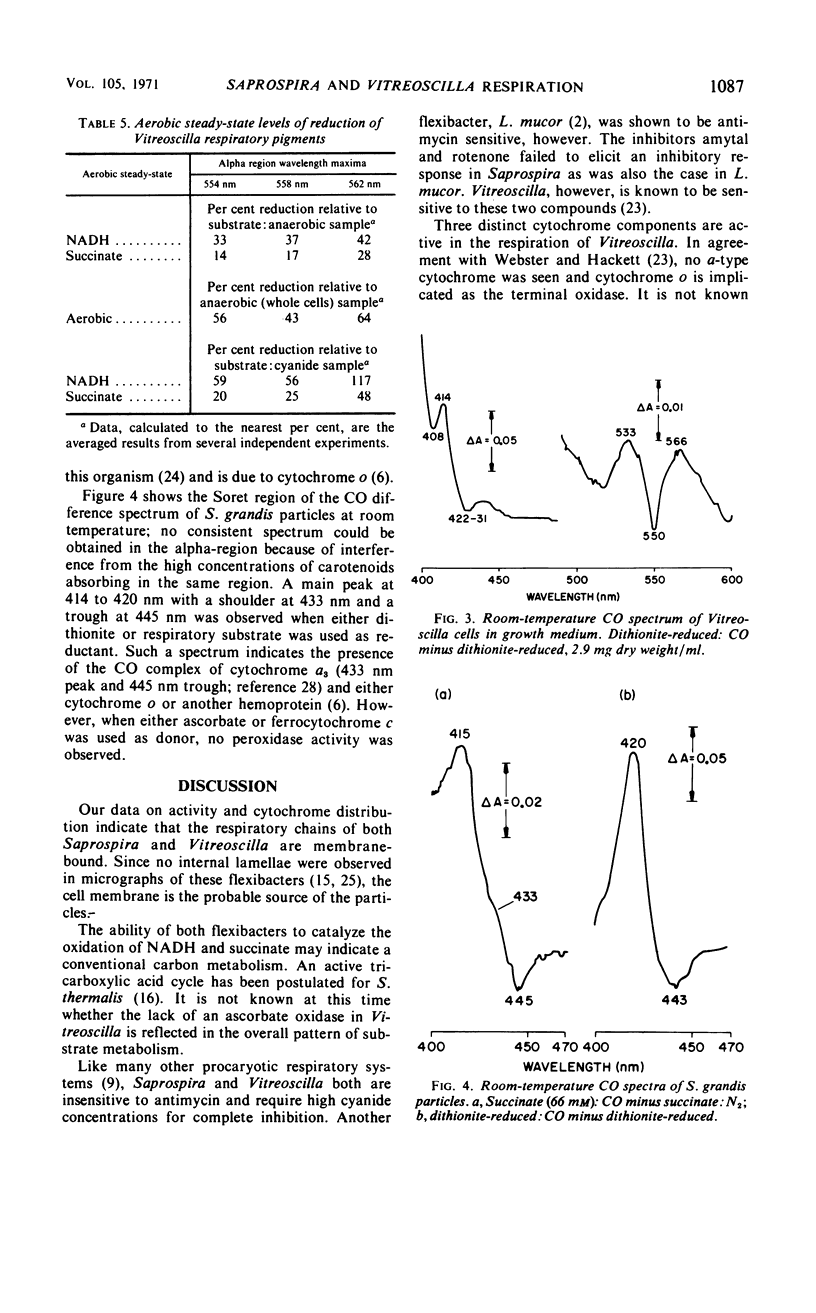
Particles from both Saprospira grandis and Vitreoscilla species, obtained by high-pressure extrusion and sonic treatment, respectively, actively catalyze the oxidation of reduced nicotinamide adenine dinucleotide (NADH) and succinate with O2. These activities are inhibited by cyanide but not by antimycin; Saprospira is also amytal- and rotenone-insensitive. Vitreoscilla preparations were unable to oxidize mammalian ferrocytochrome c and reduced tetramethyl-p-phenylenediamine, whereas the Saprospira preparations did so actively. Low-temperature (77 K) difference spectroscopy of Vitreoscilla cells and particles indicates the presence of three maxima in the cytochrome alpha-region at 554, 558, and 562 nm. All three cytochromes are active in NADH and succinate oxidation, but none is ascorbate reducible. Cytochrome o is the only CO-binding pigment present and is probably the terminal oxidase; it has properties similar to the cytochrome o isolated in solubilized form from this organism. Saprospira cells and membranes exhibit four cytochrome absorption bands whose maxima are at 550, 554, 558, and 603 nm at 77 K. The latter component has not been noted previously. NADH and succinate reduce all four cytochromes, but ascorbate reduces only the 550- and 603-nm pigments. CO spectra indicate the presence of cytochrome a,a3 which is probably the oxidase. A second CO-binding pigment is present which is not a peroxidase but may be a cytochrome.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADLEY L. B., JACOB M., JACOBS E. E., SANADI D. R. Uncoupling of oxidative phosphorylation by cadmium ion. J Biol Chem. 1956 Nov;223(1):147–156. [PubMed] [Google Scholar]
- Biggins J., Dietrich W. E., Jr Respiratory mechanisms in the Flexibacteriaceae. I. Studies on the terminal oxidase system of Leucothrix mucor. Arch Biochem Biophys. 1968 Oct;128(1):40–50. doi: 10.1016/0003-9861(68)90007-6. [DOI] [PubMed] [Google Scholar]
- Biggins J. Respiration in blue-green algae. J Bacteriol. 1969 Aug;99(2):570–575. doi: 10.1128/jb.99.2.570-575.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisalputra T., Brown D. L., Weier T. E. Possible respiratory sites in a blue-green alga Nostoc sphaericum as demonstrated by potassium tellurite and tetranitro-blue tetrazolium reduction. J Ultrastruct Res. 1969 Apr;27(2):182–197. [PubMed] [Google Scholar]
- Burton S. D., Morita R. Y., Miller W. Utilization of acetate by Beggiatoa. J Bacteriol. 1966 Mar;91(3):1192–1200. doi: 10.1128/jb.91.3.1192-1200.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTOR L. N., CHANCE B. Photochemical determinations of the oxidases of bacteria. J Biol Chem. 1959 Jun;234(6):1587–1592. [PubMed] [Google Scholar]
- Carr N. G., Hallaway M. Reduction of phenolindo-2,6-dichlorophenol in dark and light by the blue-green alga, Anabaena variabilis. J Gen Microbiol. 1965 Jun;39(3):335–344. doi: 10.1099/00221287-39-3-335. [DOI] [PubMed] [Google Scholar]
- Edelman M., Swinton D., Schiff J. A., Epstein H. T., Zeldin B. Deoxyribonucleic Acid of the blue-green algae (cyanophyta). Bacteriol Rev. 1967 Dec;31(4):315–331. doi: 10.1128/br.31.4.315-331.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory R. P. A rapid assay for peroxidase activity. Biochem J. 1966 Dec;101(3):582–583. doi: 10.1042/bj1010582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton A. A. NADH oxidase in blue-green algae. Biochem Biophys Res Commun. 1968 Sep 6;32(5):839–845. doi: 10.1016/0006-291x(68)90317-3. [DOI] [PubMed] [Google Scholar]
- LEWIN R. A. ISOLATION AND SOME PHYSIOLOGICAL FEATURES OF SAPROSPIRA THERMALIS. Can J Microbiol. 1965 Feb;11:77–86. doi: 10.1139/m65-010. [DOI] [PubMed] [Google Scholar]
- Leach C. K., Carr N. G. Oxidative phosphorylation in an extract of Anabaena variabilis. Biochem J. 1969 Mar;112(1):125–126. doi: 10.1042/bj1120125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin R. A. A classification of flexibacteria. J Gen Microbiol. 1969 Oct;58(2):189–206. doi: 10.1099/00221287-58-2-189. [DOI] [PubMed] [Google Scholar]
- Mandel M., Lewin R. A. Deoxyribonucleic acid base composition of flexibacteria. J Gen Microbiol. 1969 Oct;58(2):171–178. doi: 10.1099/00221287-58-2-171. [DOI] [PubMed] [Google Scholar]
- PRINGSHEIM E. G. The Vitreoscillaceae; a family of colourless, gliding, filamentous organisms. J Gen Microbiol. 1951 Feb;5(1):124–149. doi: 10.1099/00221287-5-1-124. [DOI] [PubMed] [Google Scholar]
- SORIANO S., LEWIN R. A. GLIDING MICROBES: SOME TAXONOMIC RECONSIDERATIONS. Antonie Van Leeuwenhoek. 1965;31:66–79. doi: 10.1007/BF02045876. [DOI] [PubMed] [Google Scholar]
- STARR T. J., KLEIN H. P. Enzymes involved in the utilization of carbohydrates by two strains of myxobacteria. Arch Mikrobiol. 1954;20(3):235–242. doi: 10.1007/BF00409623. [DOI] [PubMed] [Google Scholar]
- Webster D. A., Hackett D. P., Park R. B. The respiratory chain of colorless algae. 3. Electron microscopy. J Ultrastruct Res. 1967 Dec;21(5):514–523. doi: 10.1016/s0022-5320(67)80154-0. [DOI] [PubMed] [Google Scholar]
- Webster D. A., Hackett D. P. Respiratory chain of colorless algae. II. Cyanophyta. Plant Physiol. 1966 Apr;41(4):599–605. doi: 10.1104/pp.41.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster D. A., Hackett D. P. The purification and properties of cytochrome o from Vitreoscilla. J Biol Chem. 1966 Jul 25;241(14):3308–3315. [PubMed] [Google Scholar]
- Webster G. C., Frenkel A. W. Some Respiratory Characteristics of the Blue-Green Alga, Anabaena. Plant Physiol. 1953 Jan;28(1):63–69. doi: 10.1104/pp.28.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard J. M., Gibbs M. Purification and characterization of the fructose diphosphate aldolases from Anacystis is nidulans and Saprospira thermalis. Biochim Biophys Acta. 1968 Feb 5;151(2):438–448. doi: 10.1016/0005-2744(68)90112-5. [DOI] [PubMed] [Google Scholar]
- YONETANI T. Studies on cytochrome oxidase. I. Absolute and difference absorption spectra. J Biol Chem. 1960 Mar;235:845–852. [PubMed] [Google Scholar]


