Abstract
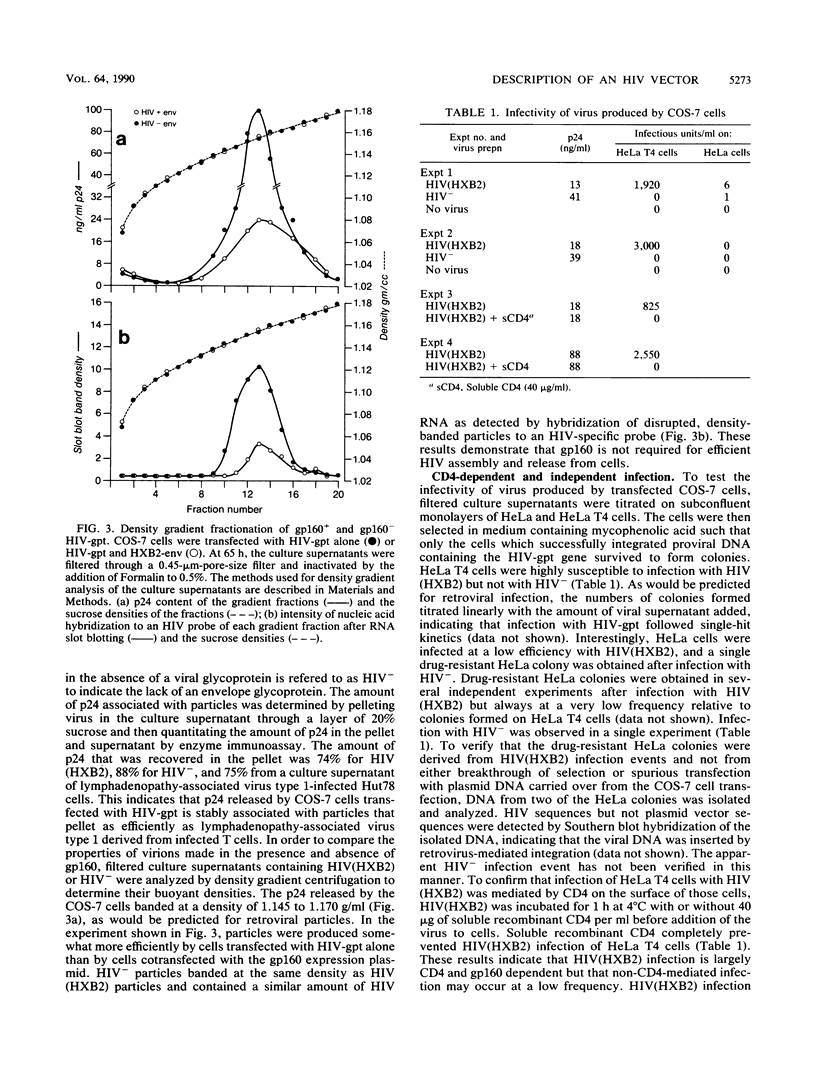
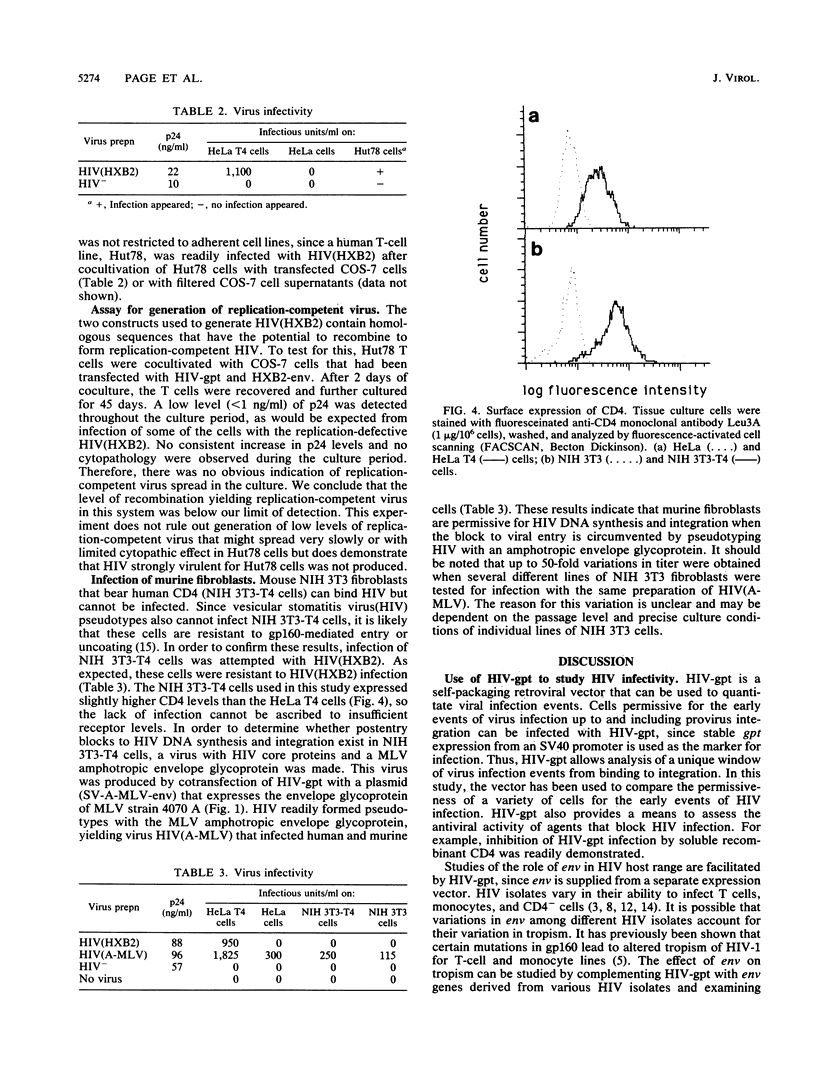
We constructed a recombinant human immunodeficiency virus (HIV) vector to facilitate studies of virus infectivity. A drug resistance gene was inserted into a gp160- HIV proviral genome such that it could be packaged into HIV virions. The HIV genome was rendered replication defective by deletion of sequences encoding gp160 and insertion of a gpt gene with a simian virus 40 promoter at the deletion site. Cotransfection of the envelope-deficient genome with a gp160 expression vector resulted in packaging of the defective HIV-gpt genome into infectious virions. The drug resistance gene was transmitted and expressed upon infection of susceptible cells, enabling their selection in mycophenolic acid. This system provides a quantitative measure of HIV infection, since each successful infection event leads to the growth of a drug-resistant colony. The HIV-gpt virus produced was tropic for CD4+ human cells and was blocked by soluble CD4. In the absence of gp160, noninfectious HIV particles were efficiently produced by cells transfected with the HIV-gpt genome. These particles packaged HIV genomic RNA and migrated to the same density as gp160-containing virions in a sucrose gradient. This demonstrates that HIV virion formation is not dependent on the presence of a viral envelope glycoprotein. Expression of a murine leukemia virus amphotropic envelope gene in cells transfected with HIV-gpt resulted in the production of virus capable of infecting both human and murine cells. These results indicate that HIV can incorporate envelope glycoproteins other than gp160 onto particles and that this can lead to altered host range. Like HIV type 1 and vesicular stomatitis virus(HIV) pseudotypes, gp-160+ HIV-gpt did not infect murine NIH 3T3 cells that bear human CD4, confirming that these cells are blocked at an early stage of HIV infection.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Besnard C., Monthioux E., Jami J. Selection against expression of the Escherichia coli gene gpt in hprt+ mouse teratocarcinoma and hybrid cells. Mol Cell Biol. 1987 Nov;7(11):4139–4141. doi: 10.1128/mcb.7.11.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Mayer C., Seto D., Tateno M., Levy J. A. Biologic features of HIV-1 that correlate with virulence in the host. Science. 1988 Apr 1;240(4848):80–82. doi: 10.1126/science.2832945. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K. Development of a sensitive quantitative focal assay for human immunodeficiency virus infectivity. J Virol. 1988 Oct;62(10):3779–3788. doi: 10.1128/jvi.62.10.3779-3788.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordonnier A., Montagnier L., Emerman M. Single amino-acid changes in HIV envelope affect viral tropism and receptor binding. Nature. 1989 Aug 17;340(6234):571–574. doi: 10.1038/340571a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Harouse J. M., Kunsch C., Hartle H. T., Laughlin M. A., Hoxie J. A., Wigdahl B., Gonzalez-Scarano F. CD4-independent infection of human neural cells by human immunodeficiency virus type 1. J Virol. 1989 Jun;63(6):2527–2533. doi: 10.1128/jvi.63.6.2527-2533.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Naturally occurring murine leukemia viruses in wild mice: characterization of a new "amphotropic" class. J Virol. 1976 Jul;19(1):19–25. doi: 10.1128/jvi.19.1.19-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helseth E., Kowalski M., Gabuzda D., Olshevsky U., Haseltine W., Sodroski J. Rapid complementation assays measuring replicative potential of human immunodeficiency virus type 1 envelope glycoprotein mutants. J Virol. 1990 May;64(5):2416–2420. doi: 10.1128/jvi.64.5.2416-2420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karacostas V., Nagashima K., Gonda M. A., Moss B. Human immunodeficiency virus-like particles produced by a vaccinia virus expression vector. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8964–8967. doi: 10.1073/pnas.86.22.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi Y., Miles S., Mitsuyasu R. T., Merrill J. E., Vinters H. V., Chen I. S. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987 May 15;236(4803):819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- Leonard J. M., Abramczuk J. W., Pezen D. S., Rutledge R., Belcher J. H., Hakim F., Shearer G., Lamperth L., Travis W., Fredrickson T. Development of disease and virus recovery in transgenic mice containing HIV proviral DNA. Science. 1988 Dec 23;242(4886):1665–1670. doi: 10.1126/science.3201255. [DOI] [PubMed] [Google Scholar]
- Li X. L., Moudgil T., Vinters H. V., Ho D. D. CD4-independent, productive infection of a neuronal cell line by human immunodeficiency virus type 1. J Virol. 1990 Mar;64(3):1383–1387. doi: 10.1128/jvi.64.3.1383-1387.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusso P., di Marzo Veronese F., Ensoli B., Franchini G., Jemma C., DeRocco S. E., Kalyanaraman V. S., Gallo R. C. Expanded HIV-1 cellular tropism by phenotypic mixing with murine endogenous retroviruses. Science. 1990 Feb 16;247(4944):848–852. doi: 10.1126/science.2305256. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Mardon G., Varmus H. E. Frameshift and intragenic suppressor mutations in a Rous sarcoma provirus suggest src encodes two proteins. Cell. 1983 Mar;32(3):871–879. doi: 10.1016/0092-8674(83)90072-7. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. L. Isolation of noninfectious particles containing Rous sarcoma virus RNA from the medium of Rous sarcoma virus-transformed nonproducer cells. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1655–1662. doi: 10.1073/pnas.57.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruta M., Murray M. J., Webb M. C., Kabat D. A murine leukemia virus mutant with a temperature-sensitive defect in membrane glycoprotein synthesis. Cell. 1979 Jan;16(1):77–88. doi: 10.1016/0092-8674(79)90189-2. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Spector D. H., Wade E., Wright D. A., Koval V., Clark C., Jaquish D., Spector S. A. Human immunodeficiency virus pseudotypes with expanded cellular and species tropism. J Virol. 1990 May;64(5):2298–2308. doi: 10.1128/jvi.64.5.2298-2308.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuve L. L., Brown-Shimer S., Pachl C., Najarian R., Dina D., Burke R. L. Structure and expression of the herpes simplex virus type 2 glycoprotein gB gene. J Virol. 1987 Feb;61(2):326–335. doi: 10.1128/jvi.61.2.326-335.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z. H., Chen S. S., Huang A. S. Phenotypic mixing between human immunodeficiency virus and vesicular stomatitis virus or herpes simplex virus. J Acquir Immune Defic Syndr. 1990;3(3):215–219. [PubMed] [Google Scholar]



