Abstract
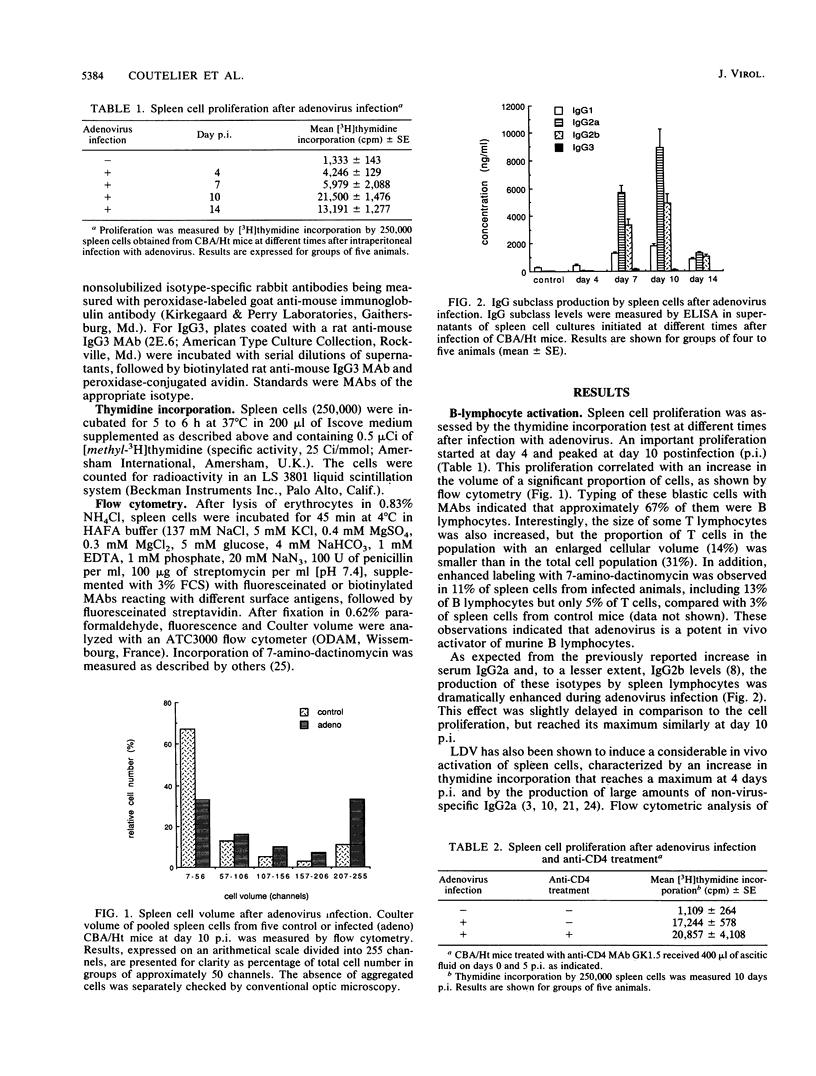
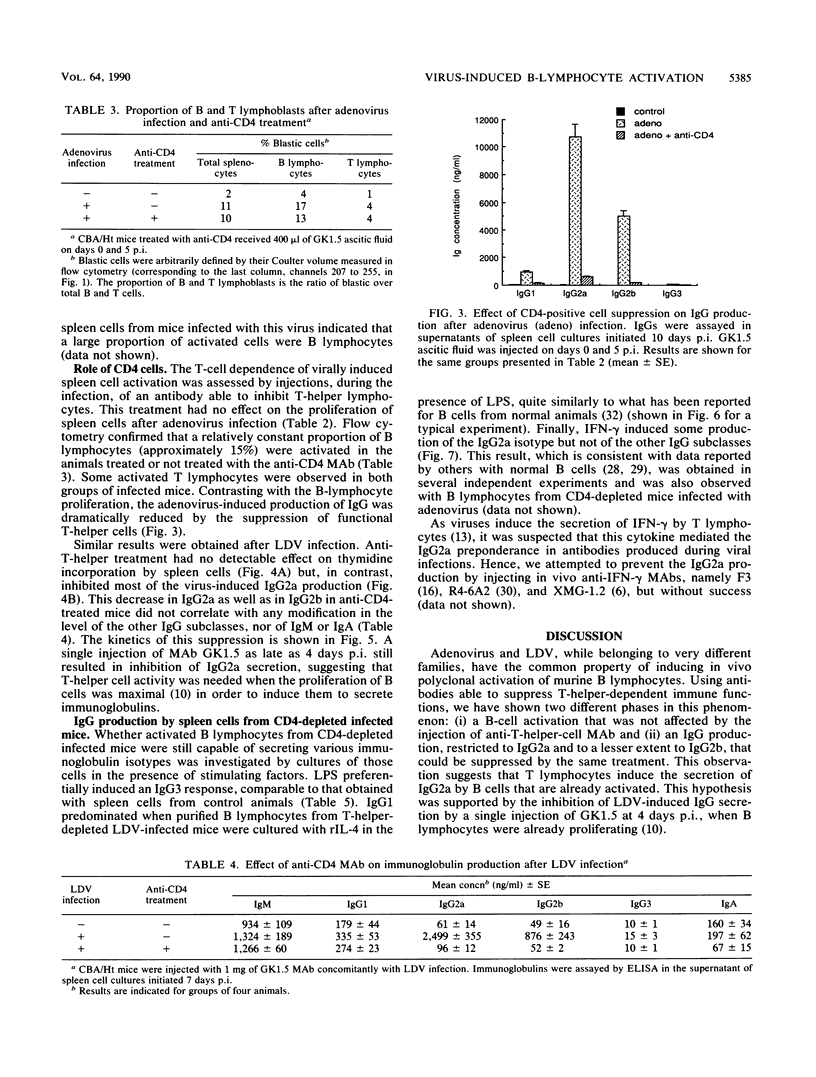
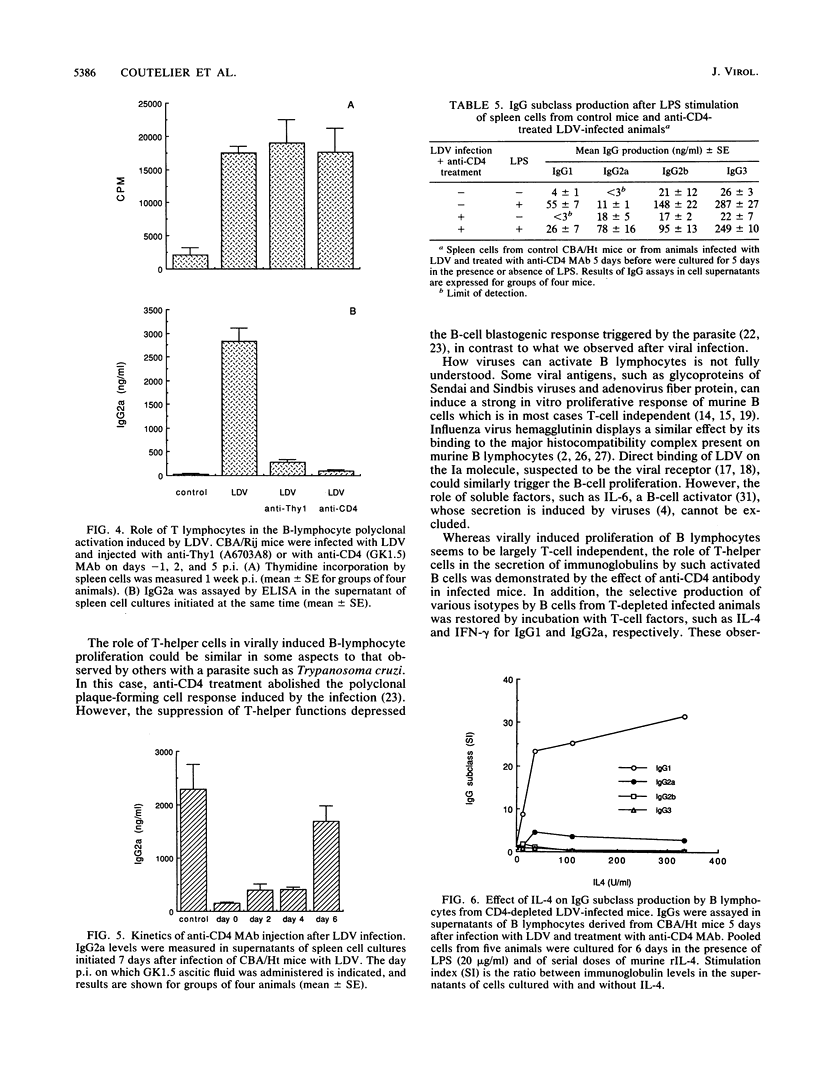
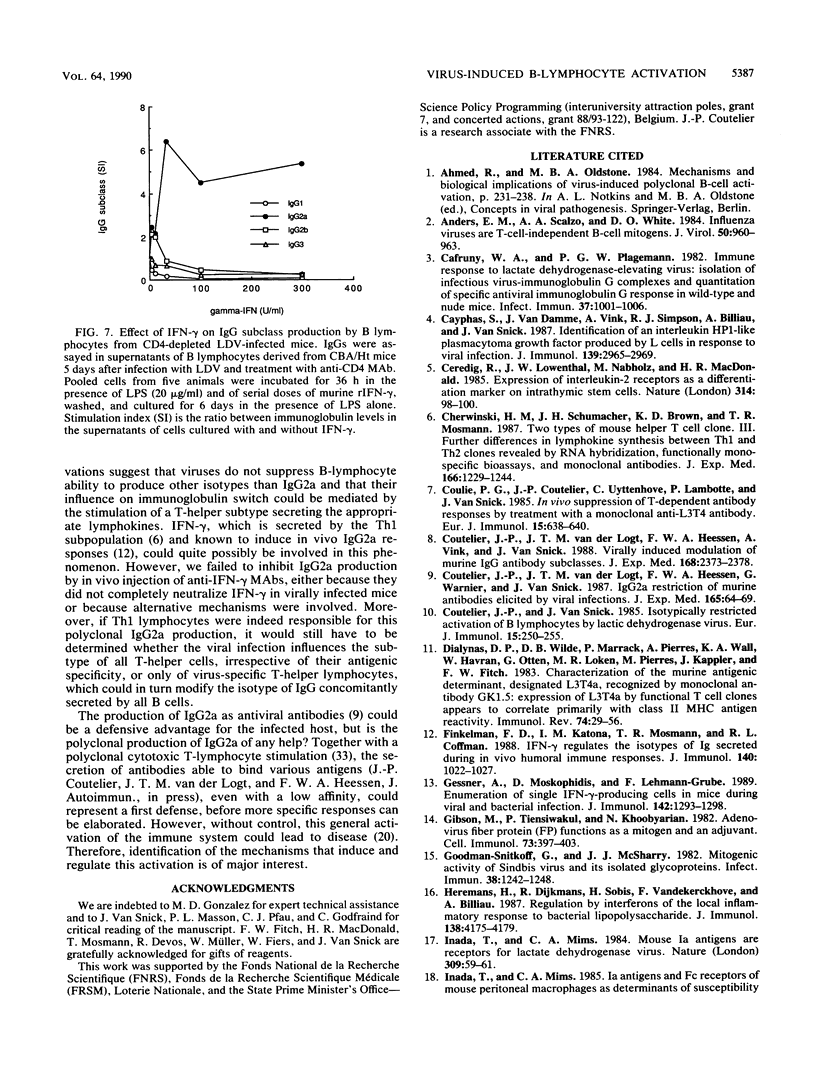
Viruses such as lactate dehydrogenase-elevating virus and adenovirus induce in vivo a polyclonal activation of murine B lymphocytes, followed by a marked increase in the production of immunoglobulin G2a (IgG2a). The role of T lymphocytes in this phenomenon was studied by injection of an anti-CD4 monoclonal antibody able to inhibit the T-helper function. This treatment profoundly depressed the production of IgG2a, whereas it had no effect on the proliferation of B cells. Activated B cells obtained from such infected and treated mice remained able to produce various immunoglobulin isotypes after exposure to an appropriate stimulus. In particular, gamma interferon, which is known to be secreted after viral infection, induced the production of IgG2a. These observations support the hypothesis that the influence of viruses on the switch of immunoglobulins is mediated by T-helper lymphocytes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders E. M., Scalzo A. A., White D. O. Influenza viruses are T cell-independent B cell mitogens. J Virol. 1984 Jun;50(3):960–963. doi: 10.1128/jvi.50.3.960-963.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafruny W. A., Plagemann P. G. Immune response to lactate dehydrogenase-elevating virus: isolation of infectious virus-immunoglobulin G complexes and quantitation of specific antiviral immunoglobulin G response in wild-type and nude mice. Infect Immun. 1982 Sep;37(3):1001–1006. doi: 10.1128/iai.37.3.1001-1006.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayphas S., Van Damme J., Vink A., Simpson R. J., Billiau A., Van Snick J. Identification of an interleukin HP1-like plasmacytoma growth factor produced by L cells in response to viral infection. J Immunol. 1987 Nov 1;139(9):2965–2969. [PubMed] [Google Scholar]
- Ceredig R., Lowenthal J. W., Nabholz M., MacDonald H. R. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985 Mar 7;314(6006):98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulie P. G., Coutelier J. P., Uyttenhove C., Lambotte P., Van Snick J. In vivo suppression of T-dependent antibody responses by treatment with a monoclonal anti-L3T4 antibody. Eur J Immunol. 1985 Jun;15(6):638–640. doi: 10.1002/eji.1830150620. [DOI] [PubMed] [Google Scholar]
- Coutelier J. P., Van Snick J. Isotypically restricted activation of B lymphocytes by lactic dehydrogenase virus. Eur J Immunol. 1985 Mar;15(3):250–255. doi: 10.1002/eji.1830150308. [DOI] [PubMed] [Google Scholar]
- Coutelier J. P., van der Logt J. T., Heessen F. W., Vink A., van Snick J. Virally induced modulation of murine IgG antibody subclasses. J Exp Med. 1988 Dec 1;168(6):2373–2378. doi: 10.1084/jem.168.6.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutelier J. P., van der Logt J. T., Heessen F. W., Warnier G., Van Snick J. IgG2a restriction of murine antibodies elicited by viral infections. J Exp Med. 1987 Jan 1;165(1):64–69. doi: 10.1084/jem.165.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Mosmann T. R., Coffman R. L. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988 Feb 15;140(4):1022–1027. [PubMed] [Google Scholar]
- Gessner A., Moskophidis D., Lehmann-Grube F. Enumeration of single IFN-gamma-producing cells in mice during viral and bacterial infection. J Immunol. 1989 Feb 15;142(4):1293–1298. [PubMed] [Google Scholar]
- Gibson M., Tiensiwakul P., Khoobyarian N. Adenovirus fiber protein (FP) functions as a mitogen and an adjuvant. Cell Immunol. 1982 Nov 1;73(2):397–403. doi: 10.1016/0008-8749(82)90466-x. [DOI] [PubMed] [Google Scholar]
- Goodman-Snitkoff G., McSharry J. J. Mitogenic activity of Sindbis virus and its isolated glycoproteins. Infect Immun. 1982 Dec;38(3):1242–1248. doi: 10.1128/iai.38.3.1242-1248.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heremans H., Dijkmans R., Sobis H., Vandekerckhove F., Billiau A. Regulation by interferons of the local inflammatory response to bacterial lipopolysaccharide. J Immunol. 1987 Jun 15;138(12):4175–4179. [PubMed] [Google Scholar]
- Inada T., Mims C. A. Mouse Ia antigens are receptors for lactate dehydrogenase virus. Nature. 1984 May 3;309(5963):59–61. doi: 10.1038/309059a0. [DOI] [PubMed] [Google Scholar]
- Kizaka S., Goodman-Snitkoff G., McSharry J. J. Sendai virus glycoproteins are T cell-dependent B cell mitogens. Infect Immun. 1983 May;40(2):592–600. doi: 10.1128/iai.40.2.592-600.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinman D. M., Morse H. C., 3rd Characteristics of B cell proliferation and activation in murine AIDS. J Immunol. 1989 Feb 15;142(4):1144–1149. [PubMed] [Google Scholar]
- Michaelides M. C., Simms E. S. Immune responses in mice infected with lactic dehydrogenase virus. III. Antibody response to a T-dependent and a T-independent antigen during acute and chronic LDV infection. Cell Immunol. 1980 Mar 15;50(2):253–260. doi: 10.1016/0008-8749(80)90280-4. [DOI] [PubMed] [Google Scholar]
- Minoprio P. M., Eisen H., Forni L., D'Imperio Lima M. R., Joskowicz M., Coutinho A. Polyclonal lymphocyte responses to murine Trypanosoma cruzi infection. I. Quantitation of both T- and B-cell responses. Scand J Immunol. 1986 Dec;24(6):661–668. doi: 10.1111/j.1365-3083.1986.tb02185.x. [DOI] [PubMed] [Google Scholar]
- Minoprio P., Eisen H., Joskowicz M., Pereira P., Coutinho A. Suppression of polyclonal antibody production in Trypanosoma cruzi-infected mice by treatment with anti-L3T4 antibodies. J Immunol. 1987 Jul 15;139(2):545–550. [PubMed] [Google Scholar]
- Notkins A. L., Mahar S., Scheele C., Goffman J. Infectious virus-antibody complex in the blood of chronically infected mice. J Exp Med. 1966 Jul 1;124(1):81–97. doi: 10.1084/jem.124.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch P. S., Torres R. M., Engel D. Simultaneous cell cycle analysis and two-color surface immunofluorescence using 7-amino-actinomycin D and single laser excitation: applications to study of cell activation and the cell cycle of murine Ly-1 B cells. J Immunol. 1986 Apr 15;136(8):2769–2775. [PubMed] [Google Scholar]
- Scalzo A. A., Anders E. M. Influenza viruses as lymphocyte mitogens. I. B cell mitogenesis by influenza A viruses of the H2 and H6 subtypes is controlled by the I-E/C subregion of the major histocompatibility complex. J Immunol. 1985 Feb;134(2):757–760. [PubMed] [Google Scholar]
- Scalzo A. A., Anders E. M. Influenza viruses as lymphocyte mitogens. II. Role of I-E molecules in B cell mitogenesis by influenza A viruses of the H2 and H6 subtypes. J Immunol. 1985 Nov;135(5):3524–3529. [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Snapper C. M., Peschel C., Paul W. E. IFN-gamma stimulates IgG2a secretion by murine B cells stimulated with bacterial lipopolysaccharide. J Immunol. 1988 Apr 1;140(7):2121–2127. [PubMed] [Google Scholar]
- Spitalny G. L., Havell E. A. Monoclonal antibody to murine gamma interferon inhibits lymphokine-induced antiviral and macrophage tumoricidal activities. J Exp Med. 1984 May 1;159(5):1560–1565. doi: 10.1084/jem.159.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink A., Coulie P. G., Wauters P., Nordan R. P., Van Snick J. B cell growth and differentiation activity of interleukin-HP1 and related murine plasmacytoma growth factors. Synergy with interleukin 1. Eur J Immunol. 1988 Apr;18(4):607–612. doi: 10.1002/eji.1830180418. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Ohara J., Myers C. D., Layton J. E., Krammer P. H., Paul W. E. Serological, biochemical, and functional identity of B cell-stimulatory factor 1 and B cell differentiation factor for IgG1. J Exp Med. 1985 Nov 1;162(5):1726–1731. doi: 10.1084/jem.162.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. Y., Dundon P. L., Nahill S. R., Welsh R. M. Virus-induced polyclonal cytotoxic T lymphocyte stimulation. J Immunol. 1989 Mar 1;142(5):1710–1718. [PubMed] [Google Scholar]


