Abstract
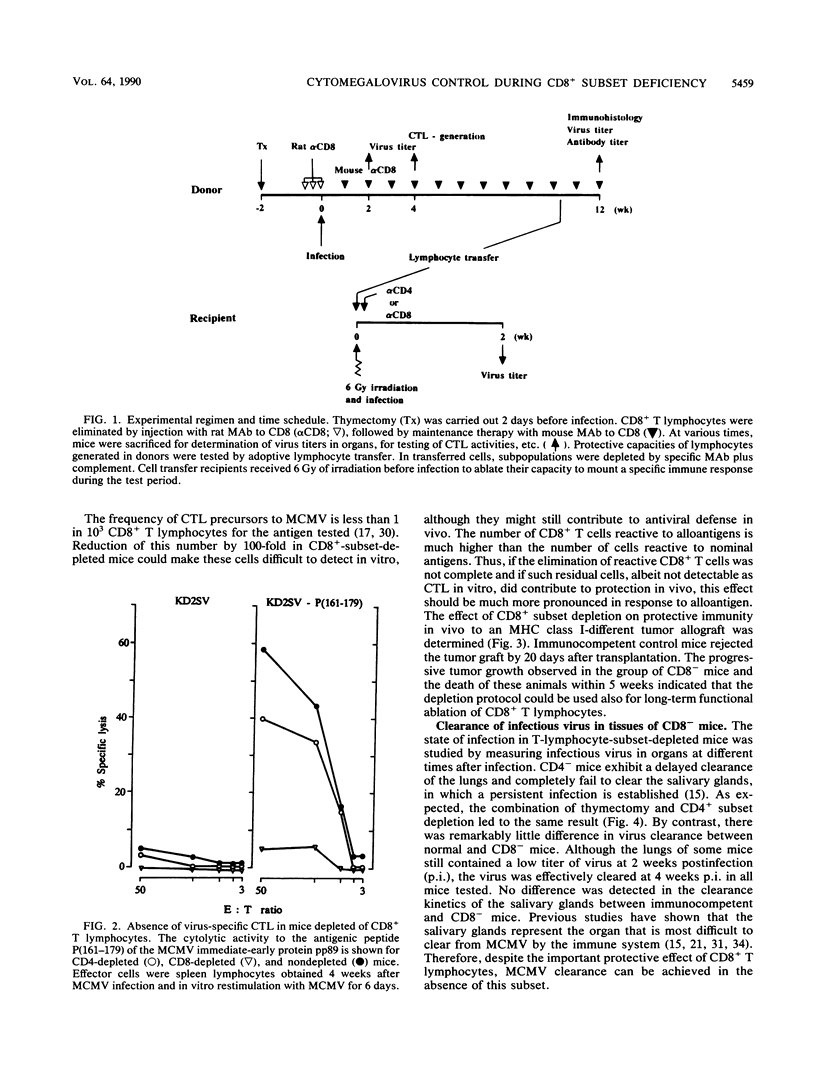
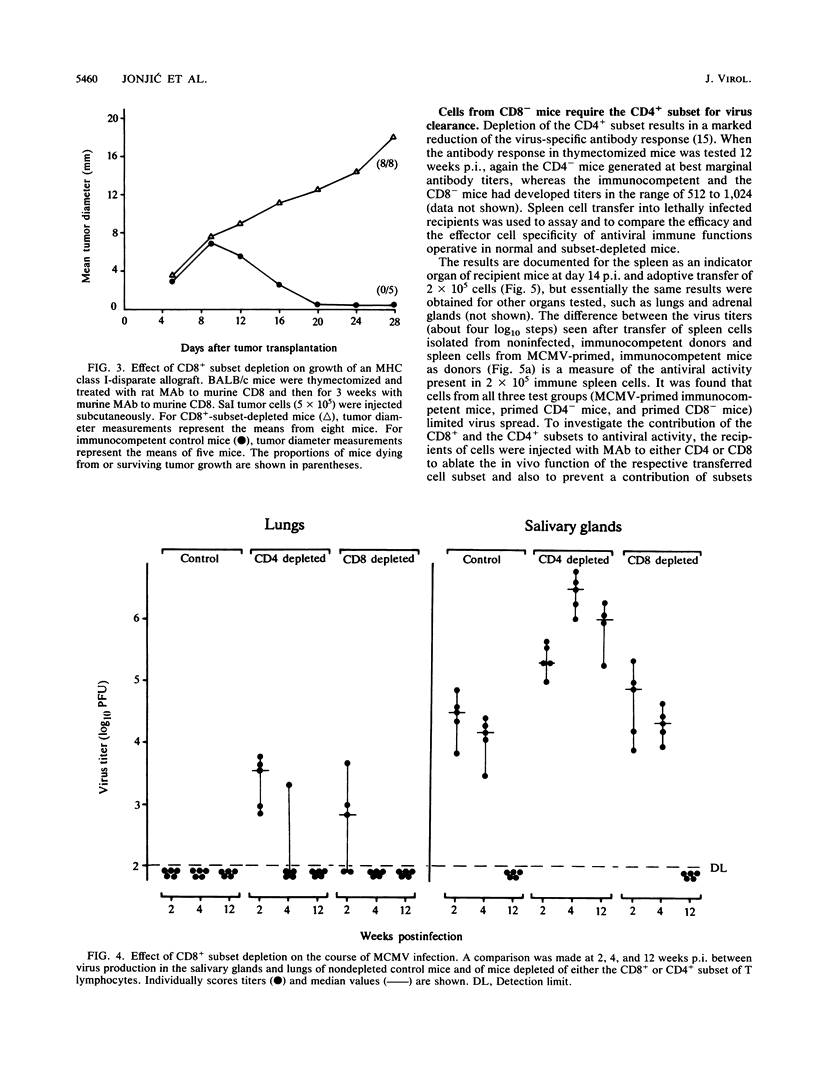
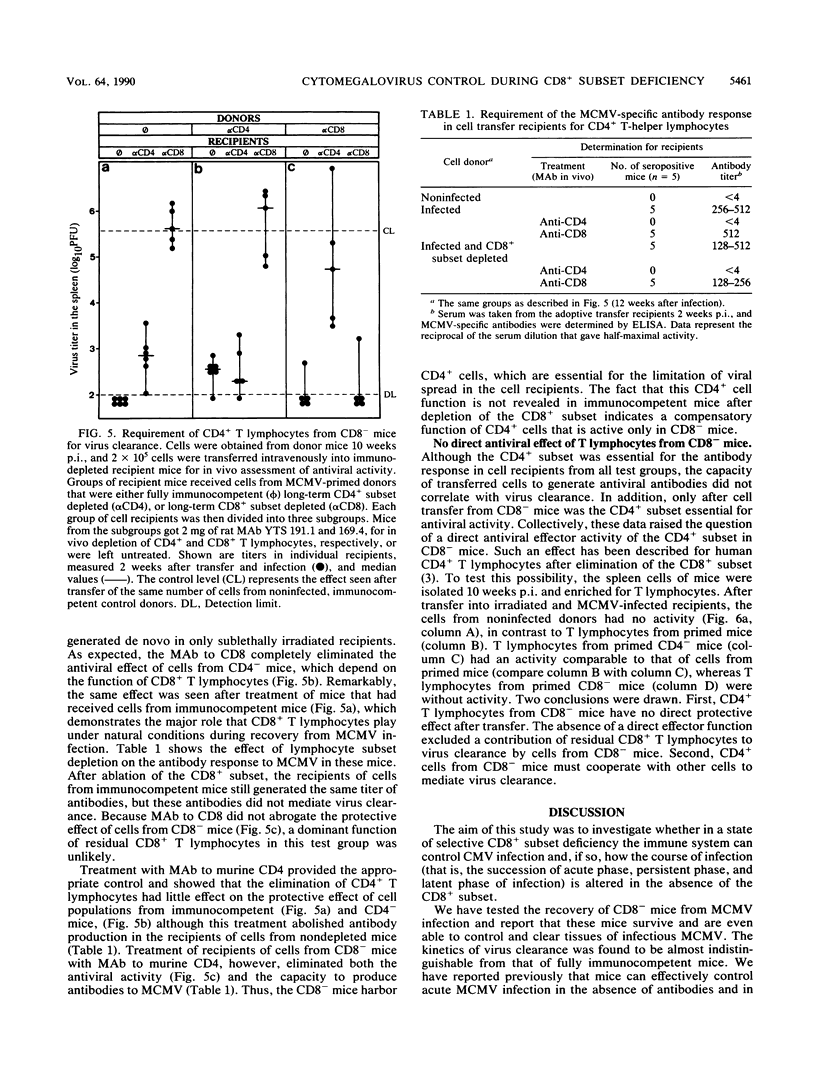
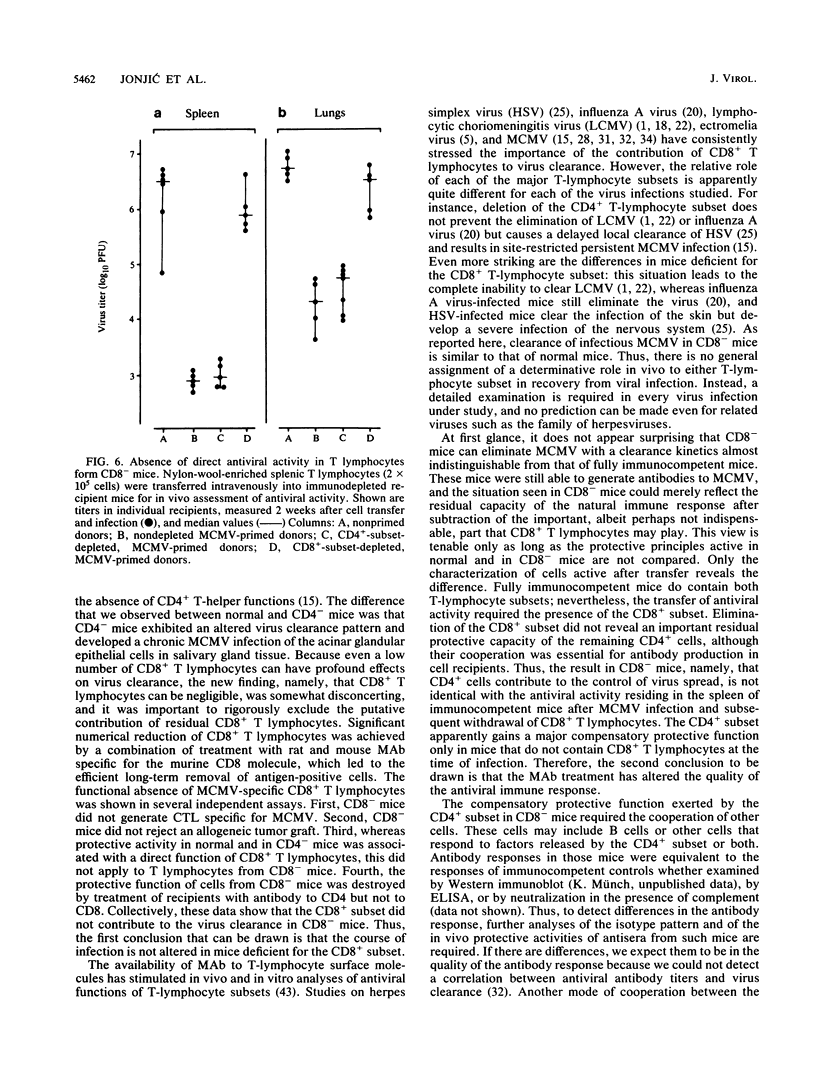
Although the relative contribution of different immune effector functions to clearing tissues of cytomegalovirus is controversial, the contribution of CD8+ T lymphocytes has generally been accepted as essential. In this report, we show that under certain conditions the CD8+ T-lymphocyte subset can be dispensable for clearance of cytomegalovirus. Mice depleted of the CD8+ T-lymphocyte subset eliminated infectious virus with a clearance kinetics similar to that of normal mice. Adoptive transfer studies revealed that the limitation of virus spread required the cooperation between the CD4+ subset and other cells. Comparison between protective functions generated in fully immunocompetent and in CD8- mice demonstrated that elimination of the CD8+ subset before infection altered the quality of the antiviral immune response. The compensatory protective activity gained by CD4+ cells in CD8- mice was absent in normal mice recovering from virus infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Butler L. D., Bhatti L. T4+ T helper cell function in vivo: differential requirement for induction of antiviral cytotoxic T-cell and antibody responses. J Virol. 1988 Jun;62(6):2102–2106. doi: 10.1128/jvi.62.6.2102-2106.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft G. J., Shellam G. R., Chalmer J. E. Genetic influences on the augmentation of natural killer (NK) cells during murine cytomegalovirus infection: correlation with patterns of resistance. J Immunol. 1981 Mar;126(3):988–994. [PubMed] [Google Scholar]
- Bourgault I., Gomez A., Gomard E., Picard F., Levy J. P., Gomrad E. A virus-specific CD4+ cell-mediated cytolytic activity revealed by CD8+ cell elimination regularly develops in uncloned human antiviral cell lines. J Immunol. 1989 Jan 1;142(1):252–256. [PubMed] [Google Scholar]
- Bukowski J. F., Warner J. F., Dennert G., Welsh R. M. Adoptive transfer studies demonstrating the antiviral effect of natural killer cells in vivo. J Exp Med. 1985 Jan 1;161(1):40–52. doi: 10.1084/jem.161.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R. M., Holmes K. L., Hügin A., Frederickson T. N., Morse H. C., 3rd Induction of cytotoxic T-cell responses in vivo in the absence of CD4 helper cells. Nature. 1987 Jul 2;328(6125):77–79. doi: 10.1038/328077a0. [DOI] [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Dallman M. J., Mason D. W., Webb M. The roles of host and donor cells in the rejection of skin allografts by T cell-deprived rats injected with syngeneic T cells. Eur J Immunol. 1982 Jun;12(6):511–518. doi: 10.1002/eji.1830120612. [DOI] [PubMed] [Google Scholar]
- Del Val M., Volkmer H., Rothbard J. B., Jonjić S., Messerle M., Schickedanz J., Reddehase M. J., Koszinowski U. H. Molecular basis for cytolytic T-lymphocyte recognition of the murine cytomegalovirus immediate-early protein pp89. J Virol. 1988 Nov;62(11):3965–3972. doi: 10.1128/jvi.62.11.3965-3972.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hudson J. B. The murine cytomegalovirus as a model for the study of viral pathogenesis and persistent infections. Arch Virol. 1979;62(1):1–29. doi: 10.1007/BF01314900. [DOI] [PubMed] [Google Scholar]
- Hämmerling G. J., Hämmerling U., Flaherty L. Qat-4 and Qat-5, new murine T-cell antigens governed by the Tla region and identified by monoclonal antibodies. J Exp Med. 1979 Jul 1;150(1):108–116. doi: 10.1084/jem.150.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonjić S., Mutter W., Weiland F., Reddehase M. J., Koszinowski U. H. Site-restricted persistent cytomegalovirus infection after selective long-term depletion of CD4+ T lymphocytes. J Exp Med. 1989 Apr 1;169(4):1199–1212. doi: 10.1084/jem.169.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonjić S., del Val M., Keil G. M., Reddehase M. J., Koszinowski U. H. A nonstructural viral protein expressed by a recombinant vaccinia virus protects against lethal cytomegalovirus infection. J Virol. 1988 May;62(5):1653–1658. doi: 10.1128/jvi.62.5.1653-1658.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil G. M., Ebeling-Keil A., Koszinowski U. H. Sequence and structural organization of murine cytomegalovirus immediate-early gene 1. J Virol. 1987 Jun;61(6):1901–1908. doi: 10.1128/jvi.61.6.1901-1908.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszinowski U. H., Keil G. M., Schwarz H., Schickedanz J., Reddehase M. J. A nonstructural polypeptide encoded by immediate-early transcription unit 1 of murine cytomegalovirus is recognized by cytolytic T lymphocytes. J Exp Med. 1987 Jul 1;166(1):289–294. doi: 10.1084/jem.166.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist T. P., Cobbold S. P., Waldmann H., Aguet M., Zinkernagel R. M. Functional analysis of T lymphocyte subsets in antiviral host defense. J Immunol. 1987 Apr 1;138(7):2278–2281. [PubMed] [Google Scholar]
- Leung K. N., Nash A. A., Sia D. Y., Wildy P. Clonal analysis of T-cell responses to herpes simplex virus: isolation, characterization and antiviral properties of an antigen-specific helper T-cell clone. Immunology. 1984 Dec;53(4):623–633. [PMC free article] [PubMed] [Google Scholar]
- Lightman S., Cobbold S., Waldmann H., Askonas B. A. Do L3T4+ T cells act as effector cells in protection against influenza virus infection. Immunology. 1987 Sep;62(1):139–144. [PMC free article] [PubMed] [Google Scholar]
- Mayo D. R., Armstrong J. A., Ho M. Reactivation of murine cytomegalovirus by cyclophosphamide. Nature. 1977 Jun 23;267(5613):721–723. doi: 10.1038/267721a0. [DOI] [PubMed] [Google Scholar]
- Moskophidis D., Cobbold S. P., Waldmann H., Lehmann-Grube F. Mechanism of recovery from acute virus infection: treatment of lymphocytic choriomeningitis virus-infected mice with monoclonal antibodies reveals that Lyt-2+ T lymphocytes mediate clearance of virus and regulate the antiviral antibody response. J Virol. 1987 Jun;61(6):1867–1874. doi: 10.1128/jvi.61.6.1867-1874.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Mutter W., Reddehase M. J., Busch F. W., Bühring H. J., Koszinowski U. H. Failure in generating hemopoietic stem cells is the primary cause of death from cytomegalovirus disease in the immunocompromised host. J Exp Med. 1988 May 1;167(5):1645–1658. doi: 10.1084/jem.167.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Kirmani N., Rook A. H., Manischewitz J. F., Jackson L., Moreschi G., Santos G. W., Saral R., Burns W. H. Cytotoxic t cells in cytomegalovirus infection: HLA-restricted T-lymphocyte and non-T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone-marrow-transplant recipients. N Engl J Med. 1982 Jul 1;307(1):7–13. doi: 10.1056/NEJM198207013070102. [DOI] [PubMed] [Google Scholar]
- Quinnan G. V., Manischewitz J. E. The role of natural killer cells and antibody-dependent cell-mediated cytotoxicity during murine cytomegalovirus infection. J Exp Med. 1979 Dec 1;150(6):1549–1554. doi: 10.1084/jem.150.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddehase M. J., Jonjić S., Weiland F., Mutter W., Koszinowski U. H. Adoptive immunotherapy of murine cytomegalovirus adrenalitis in the immunocompromised host: CD4-helper-independent antiviral function of CD8-positive memory T lymphocytes derived from latently infected donors. J Virol. 1988 Mar;62(3):1061–1065. doi: 10.1128/jvi.62.3.1061-1065.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddehase M. J., Keil G. M., Koszinowski U. H. The cytolytic T lymphocyte response to the murine cytomegalovirus. I. Distinct maturation stages of cytolytic T lymphocytes constitute the cellular immune response during acute infection of mice with the murine cytomegalovirus. J Immunol. 1984 Jan;132(1):482–489. [PubMed] [Google Scholar]
- Reddehase M. J., Koszinowski U. H. Significance of herpesvirus immediate early gene expression in cellular immunity to cytomegalovirus infection. Nature. 1984 Nov 22;312(5992):369–371. doi: 10.1038/312369a0. [DOI] [PubMed] [Google Scholar]
- Reddehase M. J., Mutter W., Koszinowski U. H. In vivo application of recombinant interleukin 2 in the immunotherapy of established cytomegalovirus infection. J Exp Med. 1987 Mar 1;165(3):650–656. doi: 10.1084/jem.165.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddehase M. J., Mutter W., Münch K., Bühring H. J., Koszinowski U. H. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol. 1987 Oct;61(10):3102–3108. doi: 10.1128/jvi.61.10.3102-3108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddehase M. J., Rothbard J. B., Koszinowski U. H. A pentapeptide as minimal antigenic determinant for MHC class I-restricted T lymphocytes. Nature. 1989 Feb 16;337(6208):651–653. doi: 10.1038/337651a0. [DOI] [PubMed] [Google Scholar]
- Reddehase M. J., Weiland F., Münch K., Jonjic S., Lüske A., Koszinowski U. H. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J Virol. 1985 Aug;55(2):264–273. doi: 10.1128/jvi.55.2.264-273.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seid J. M., Liberto M., Bonina L., Leung K. N., Nash A. A. T cell-macrophage interactions in the immune response to herpes simplex virus: the significance of interferon-gamma. J Gen Virol. 1986 Dec;67(Pt 12):2799–2802. doi: 10.1099/0022-1317-67-12-2799. [DOI] [PubMed] [Google Scholar]
- Shanley J. D. In vivo administration of monoclonal antibody to the NK 1.1 antigen of natural killer cells: effect on acute murine cytomegalovirus infection. J Med Virol. 1990 Jan;30(1):58–60. doi: 10.1002/jmv.1890300113. [DOI] [PubMed] [Google Scholar]
- Shanley J. D., Jordan M. C., Stevens J. G. Modification by adoptive humoral immunity of murine cytomegalovirus infection. J Infect Dis. 1981 Feb;143(2):231–237. doi: 10.1093/infdis/143.2.231. [DOI] [PubMed] [Google Scholar]
- Shanley J. D. Modification of acute murine cytomegalovirus adrenal gland infection by adoptive spleen cell transfer. J Virol. 1987 Jan;61(1):23–28. doi: 10.1128/jvi.61.1.23-28.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellam G. R., Allan J. E., Papadimitriou J. M., Bancroft G. J. Increased susceptibility to cytomegalovirus infection in beige mutant mice. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5104–5108. doi: 10.1073/pnas.78.8.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. M., Stuart F. P., Wemhoff G. A., Quintáns J., Fitch F. W. Cellular pathways for rejection of class-I-MHC--disparate skin and tumor allografts. Transplantation. 1988 Jan;45(1):168–175. doi: 10.1097/00007890-198801000-00036. [DOI] [PubMed] [Google Scholar]
- Sullivan K. M. Immunoglobulin therapy in bone marrow transplantation. Am J Med. 1987 Oct 23;83(4A):34–45. doi: 10.1016/0002-9343(87)90549-3. [DOI] [PubMed] [Google Scholar]
- Volkmer H., Bertholet C., Jonjić S., Wittek R., Koszinowski U. H. Cytolytic T lymphocyte recognition of the murine cytomegalovirus nonstructural immediate-early protein pp89 expressed by recombinant vaccinia virus. J Exp Med. 1987 Sep 1;166(3):668–677. doi: 10.1084/jem.166.3.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann H. Manipulation of T-cell responses with monoclonal antibodies. Annu Rev Immunol. 1989;7:407–444. doi: 10.1146/annurev.iy.07.040189.002203. [DOI] [PubMed] [Google Scholar]


