Abstract
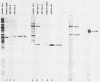
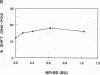
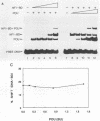
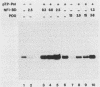
Initiation of adenovirus DNA replication is strongly enhanced by two transcription factors, nuclear factor I (NFI) and nuclear factor III (NFIII/oct-1). These proteins bind to two closely spaced recognition sequences in the origin. We produced NFI and NFIII/oct-1, as well as their biologically active, replication-competent DNA-binding domains (NFI-BD and the POU domain), in a vaccinia virus expression system and purified these polypeptides to apparent homogeneity. By DNase I footprinting and gel retardation, we show that the two proteins, as well as their purified DNA-binding domains, bind independently and without cooperative effects to their recognition sequences. By using a reconstituted system consisting of the purified viral proteins (precursor terminal protein-DNA polymerase complex (pTP-pol) and DNA-binding protein, we show that NFIII/oct-1 or the POU domain stimulates DNA replication in the absence of NFI or NFI-BD and vice versa. When added together, the enhancing effect of the two transcription factors was independent and nonsynergistic. Interestingly, stimulation by NFI or NFI-BD was strongly dependent on the concentration of the pTP-pol complex. At low pTP-pol concentrations, NFI or NFI-BD stimulated up to 50-fold, while at high concentrations, the stimulation was less than twofold, indicating that the need for NFI can be overcome by high pTP-pol concentrations. In contrast, stimulation by NFIII/oct-1 or the POU domain was much less dependent on the pTP-pol concentration. These data support a model in which NFI enhances initiation through an interaction with pTP-pol. Glutaraldehyde cross-linking experiments indicate contacts between pTP-pol and NFI but not NFIII/oct-1. The site of interaction is located in the NFI-BD domain.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Shneidman P. S., Hurwitz J. Reconstruction of adenovirus replication origins with a human nuclear factor I binding site. J Biol Chem. 1986 Mar 5;261(7):3339–3346. [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J. Animal virus DNA replication. Annu Rev Biochem. 1989;58:671–717. doi: 10.1146/annurev.bi.58.070189.003323. [DOI] [PubMed] [Google Scholar]
- Cleat P. H., Hay R. T. Co-operative interactions between NFI and the adenovirus DNA binding protein at the adenovirus origin of replication. EMBO J. 1989 Jun;8(6):1841–1848. doi: 10.1002/j.1460-2075.1989.tb03579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courey A. J., Holtzman D. A., Jackson S. P., Tjian R. Synergistic activation by the glutamine-rich domains of human transcription factor Sp1. Cell. 1989 Dec 1;59(5):827–836. doi: 10.1016/0092-8674(89)90606-5. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L. Transcriptional elements as components of eukaryotic origins of DNA replication. Cell. 1988 Mar 11;52(5):635–638. doi: 10.1016/0092-8674(88)90398-4. [DOI] [PubMed] [Google Scholar]
- Dynan W. S. Modularity in promoters and enhancers. Cell. 1989 Jul 14;58(1):1–4. doi: 10.1016/0092-8674(89)90393-0. [DOI] [PubMed] [Google Scholar]
- Fletcher C., Heintz N., Roeder R. G. Purification and characterization of OTF-1, a transcription factor regulating cell cycle expression of a human histone H2b gene. Cell. 1987 Dec 4;51(5):773–781. doi: 10.1016/0092-8674(87)90100-0. [DOI] [PubMed] [Google Scholar]
- Friefeld B. R., Korn R., de Jong P. J., Sninsky J. J., Horwitz M. S. The 140-kDa adenovirus DNA polymerase is recognized by antibodies to Escherichia coli-synthesized determinants predicted from an open reading frame on the adenovirus genome. Proc Natl Acad Sci U S A. 1985 May;82(9):2652–2656. doi: 10.1073/pnas.82.9.2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerster T., Roeder R. G. A herpesvirus trans-activating protein interacts with transcription factor OTF-1 and other cellular proteins. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6347–6351. doi: 10.1073/pnas.85.17.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goding C. R., O'Hare P. Herpes simplex virus Vmw65-octamer binding protein interaction: a paradigm for combinatorial control of transcription. Virology. 1989 Dec;173(2):363–367. doi: 10.1016/0042-6822(89)90548-5. [DOI] [PubMed] [Google Scholar]
- Gounari F., De Francesco R., Schmitt J., van der Vliet P., Cortese R., Stunnenberg H. Amino-terminal domain of NF1 binds to DNA as a dimer and activates adenovirus DNA replication. EMBO J. 1990 Feb;9(2):559–566. doi: 10.1002/j.1460-2075.1990.tb08143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay R. T. Origin of adenovirus DNA replication. Role of the nuclear factor I binding site in vivo. J Mol Biol. 1985 Nov 5;186(1):129–136. doi: 10.1016/0022-2836(85)90263-3. [DOI] [PubMed] [Google Scholar]
- Hay R. T., Russell W. C. Recognition mechanisms in the synthesis of animal virus DNA. Biochem J. 1989 Feb 15;258(1):3–16. doi: 10.1042/bj2580003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Rosenfeld P. J., Kelly T. J., Tjian R. A cellular DNA-binding protein that activates eukaryotic transcription and DNA replication. Cell. 1987 Jan 16;48(1):79–89. doi: 10.1016/0092-8674(87)90358-8. [DOI] [PubMed] [Google Scholar]
- Kemler I., Schreiber E., Müller M. M., Matthias P., Schaffner W. Octamer transcription factors bind to two different sequence motifs of the immunoglobulin heavy chain promoter. EMBO J. 1989 Jul;8(7):2001–2008. doi: 10.1002/j.1460-2075.1989.tb03607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBowitz J. H., Clerc R. G., Brenowitz M., Sharp P. A. The Oct-2 protein binds cooperatively to adjacent octamer sites. Genes Dev. 1989 Oct;3(10):1625–1638. doi: 10.1101/gad.3.10.1625. [DOI] [PubMed] [Google Scholar]
- Leegwater P. A., van Driel W., van der Vliet P. C. Recognition site of nuclear factor I, a sequence-specific DNA-binding protein from HeLa cells that stimulates adenovirus DNA replication. EMBO J. 1985 Jun;4(6):1515–1521. doi: 10.1002/j.1460-2075.1985.tb03811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaj I. W., Lienhard S., Jiricny J., De Robertis E. M. An enhancer-like sequence within the Xenopus U2 gene promoter facilitates the formation of stable transcription complexes. Nature. 1985 Jul 11;316(6024):163–167. doi: 10.1038/316163a0. [DOI] [PubMed] [Google Scholar]
- Meisterernst M., Rogge L., Foeckler R., Karaghiosoff M., Winnacker E. L. Structural and functional organization of a porcine gene coding for nuclear factor I. Biochemistry. 1989 Oct 3;28(20):8191–8200. doi: 10.1021/bi00446a034. [DOI] [PubMed] [Google Scholar]
- Mermod N., O'Neill E. A., Kelly T. J., Tjian R. The proline-rich transcriptional activator of CTF/NF-I is distinct from the replication and DNA binding domain. Cell. 1989 Aug 25;58(4):741–753. doi: 10.1016/0092-8674(89)90108-6. [DOI] [PubMed] [Google Scholar]
- Mul Y. M., van Miltenburg R. T., De Clercq E., van der Vliet P. C. Mechanism of inhibition of adenovirus DNA replication by the acyclic nucleoside triphosphate analogue (S)-HPMPApp: influence of the adenovirus DNA binding protein. Nucleic Acids Res. 1989 Nov 25;17(22):8917–8929. doi: 10.1093/nar/17.22.8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Enomoto T., Lichy J. H., Hurwitz J. Adenovirus DNA replication in vitro: identification of a host factor that stimulates synthesis of the preterminal protein-dCMP complex. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6438–6442. doi: 10.1073/pnas.79.21.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare P., Goding C. R., Haigh A. Direct combinatorial interaction between a herpes simplex virus regulatory protein and a cellular octamer-binding factor mediates specific induction of virus immediate-early gene expression. EMBO J. 1988 Dec 20;7(13):4231–4238. doi: 10.1002/j.1460-2075.1988.tb03320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill E. A., Fletcher C., Burrow C. R., Heintz N., Roeder R. G., Kelly T. J. Transcription factor OTF-1 is functionally identical to the DNA replication factor NF-III. Science. 1988 Sep 2;241(4870):1210–1213. doi: 10.1126/science.3413485. [DOI] [PubMed] [Google Scholar]
- Paonessa G., Gounari F., Frank R., Cortese R. Purification of a NF1-like DNA-binding protein from rat liver and cloning of the corresponding cDNA. EMBO J. 1988 Oct;7(10):3115–3123. doi: 10.1002/j.1460-2075.1988.tb03178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poellinger L., Roeder R. G. Octamer transcription factors 1 and 2 each bind to two different functional elements in the immunoglobulin heavy-chain promoter. Mol Cell Biol. 1989 Feb;9(2):747–756. doi: 10.1128/mcb.9.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruijn G. J., van Driel W., van Miltenburg R. T., van der Vliet P. C. Promoter and enhancer elements containing a conserved sequence motif are recognized by nuclear factor III, a protein stimulating adenovirus DNA replication. EMBO J. 1987 Dec 1;6(12):3771–3778. doi: 10.1002/j.1460-2075.1987.tb02712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruijn G. J., van Driel W., van der Vliet P. C. Nuclear factor III, a novel sequence-specific DNA-binding protein from HeLa cells stimulating adenovirus DNA replication. Nature. 1986 Aug 14;322(6080):656–659. doi: 10.1038/322656a0. [DOI] [PubMed] [Google Scholar]
- Pruijn G. J., van Miltenburg R. T., Claessens J. A., van der Vliet P. C. Interaction between the octamer-binding protein nuclear factor III and the adenovirus origin of DNA replication. J Virol. 1988 Sep;62(9):3092–3102. doi: 10.1128/jvi.62.9.3092-3102.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruijn J. M., van der Vliet P. C., Dathan N. A., Mattaj I. W. Anti-OTF-1 antibodies inhibit NFIII stimulation of in vitro adenovirus DNA replication. Nucleic Acids Res. 1989 Mar 11;17(5):1845–1863. doi: 10.1093/nar/17.5.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld P. J., O'Neill E. A., Wides R. J., Kelly T. J. Sequence-specific interactions between cellular DNA-binding proteins and the adenovirus origin of DNA replication. Mol Cell Biol. 1987 Feb;7(2):875–886. doi: 10.1128/mcb.7.2.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro C., Mermod N., Andrews P. C., Tjian R. A family of human CCAAT-box-binding proteins active in transcription and DNA replication: cloning and expression of multiple cDNAs. Nature. 1988 Jul 21;334(6179):218–224. doi: 10.1038/334218a0. [DOI] [PubMed] [Google Scholar]
- Stern S., Tanaka M., Herr W. The Oct-1 homoeodomain directs formation of a multiprotein-DNA complex with the HSV transactivator VP16. Nature. 1989 Oct 19;341(6243):624–630. doi: 10.1038/341624a0. [DOI] [PubMed] [Google Scholar]
- Stillman B. Initiation of eukaryotic DNA replication in vitro. Annu Rev Cell Biol. 1989;5:197–245. doi: 10.1146/annurev.cb.05.110189.001213. [DOI] [PubMed] [Google Scholar]
- Stuiver M. H., van der Vliet P. C. Adenovirus DNA-binding protein forms a multimeric protein complex with double-stranded DNA and enhances binding of nuclear factor I. J Virol. 1990 Jan;64(1):379–386. doi: 10.1128/jvi.64.1.379-386.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stunnenberg H. G., Lange H., Philipson L., van Miltenburg R. T., van der Vliet P. C. High expression of functional adenovirus DNA polymerase and precursor terminal protein using recombinant vaccinia virus. Nucleic Acids Res. 1988 Mar 25;16(6):2431–2444. doi: 10.1093/nar/16.6.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm R. A., Das G., Herr W. The ubiquitous octamer-binding protein Oct-1 contains a POU domain with a homeo box subdomain. Genes Dev. 1988 Dec;2(12A):1582–1599. doi: 10.1101/gad.2.12a.1582. [DOI] [PubMed] [Google Scholar]
- Sturm R. A., Herr W. The POU domain is a bipartite DNA-binding structure. Nature. 1988 Dec 8;336(6199):601–604. doi: 10.1038/336601a0. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Herr W. Differential transcriptional activation by Oct-1 and Oct-2: interdependent activation domains induce Oct-2 phosphorylation. Cell. 1990 Feb 9;60(3):375–386. doi: 10.1016/0092-8674(90)90589-7. [DOI] [PubMed] [Google Scholar]
- Triezenberg S. J., Kingsbury R. C., McKnight S. L. Functional dissection of VP16, the trans-activator of herpes simplex virus immediate early gene expression. Genes Dev. 1988 Jun;2(6):718–729. doi: 10.1101/gad.2.6.718. [DOI] [PubMed] [Google Scholar]
- Tsernoglou D., Tucker A. D., Van der Vliet P. C. Crystallization of a fragment of the adenovirus DNA binding protein. J Mol Biol. 1984 Jan 15;172(2):237–239. doi: 10.1016/s0022-2836(84)80042-x. [DOI] [PubMed] [Google Scholar]
- Verrijzer C. P., Kal A. J., Van der Vliet P. C. The DNA binding domain (POU domain) of transcription factor oct-1 suffices for stimulation of DNA replication. EMBO J. 1990 Jun;9(6):1883–1888. doi: 10.1002/j.1460-2075.1990.tb08314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Pearson G. D. Adenovirus sequences required for replication in vivo. Nucleic Acids Res. 1985 Jul 25;13(14):5173–5187. doi: 10.1093/nar/13.14.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wides R. J., Challberg M. D., Rawlins D. R., Kelly T. J. Adenovirus origin of DNA replication: sequence requirements for replication in vitro. Mol Cell Biol. 1987 Feb;7(2):864–874. doi: 10.1128/mcb.7.2.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L. J., Padmanabhan R. Nuclear transport of adenovirus DNA polymerase is facilitated by interaction with preterminal protein. Cell. 1988 Dec 23;55(6):1005–1015. doi: 10.1016/0092-8674(88)90245-0. [DOI] [PubMed] [Google Scholar]
- de Vries E., van Driel W., Tromp M., van Boom J., van der Vliet P. C. Adenovirus DNA replication in vitro: site-directed mutagenesis of the nuclear factor I binding site of the Ad2 origin. Nucleic Acids Res. 1985 Jul 11;13(13):4935–4952. doi: 10.1093/nar/13.13.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries E., van Driel W., van den Heuvel S. J., van der Vliet P. C. Contactpoint analysis of the HeLa nuclear factor I recognition site reveals symmetrical binding at one side of the DNA helix. EMBO J. 1987 Jan;6(1):161–168. doi: 10.1002/j.1460-2075.1987.tb04734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]