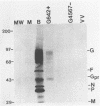
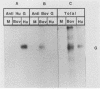
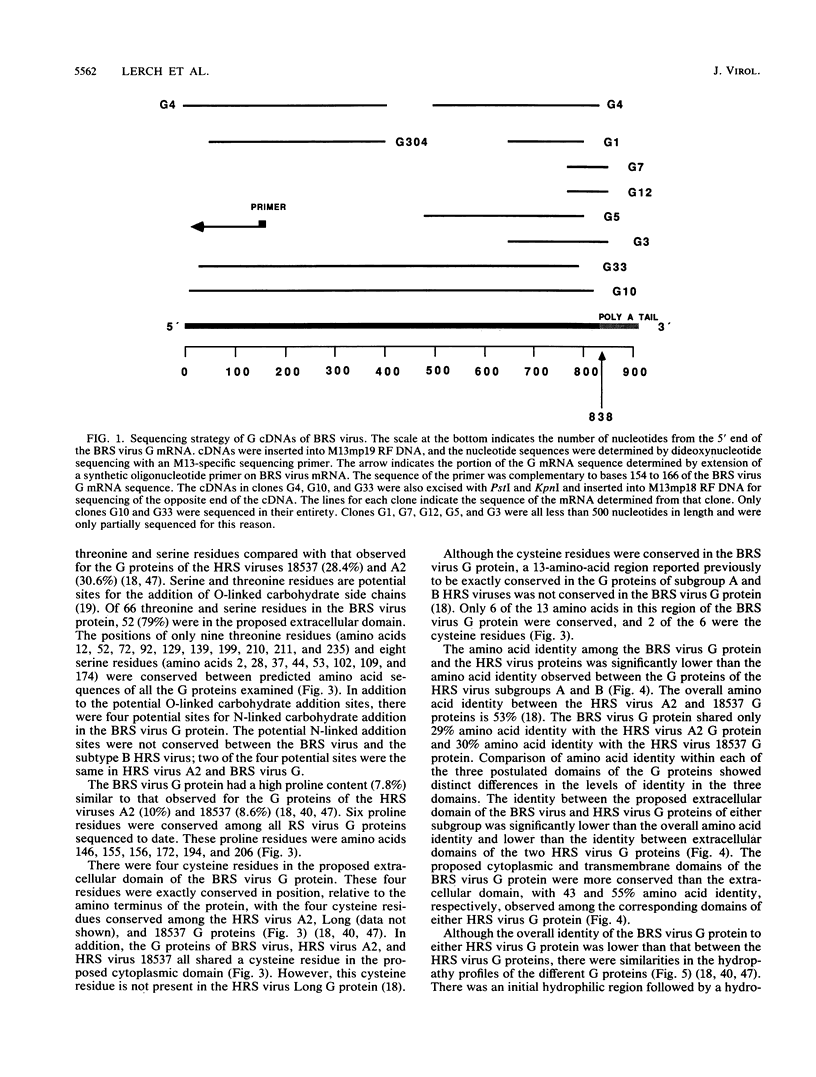
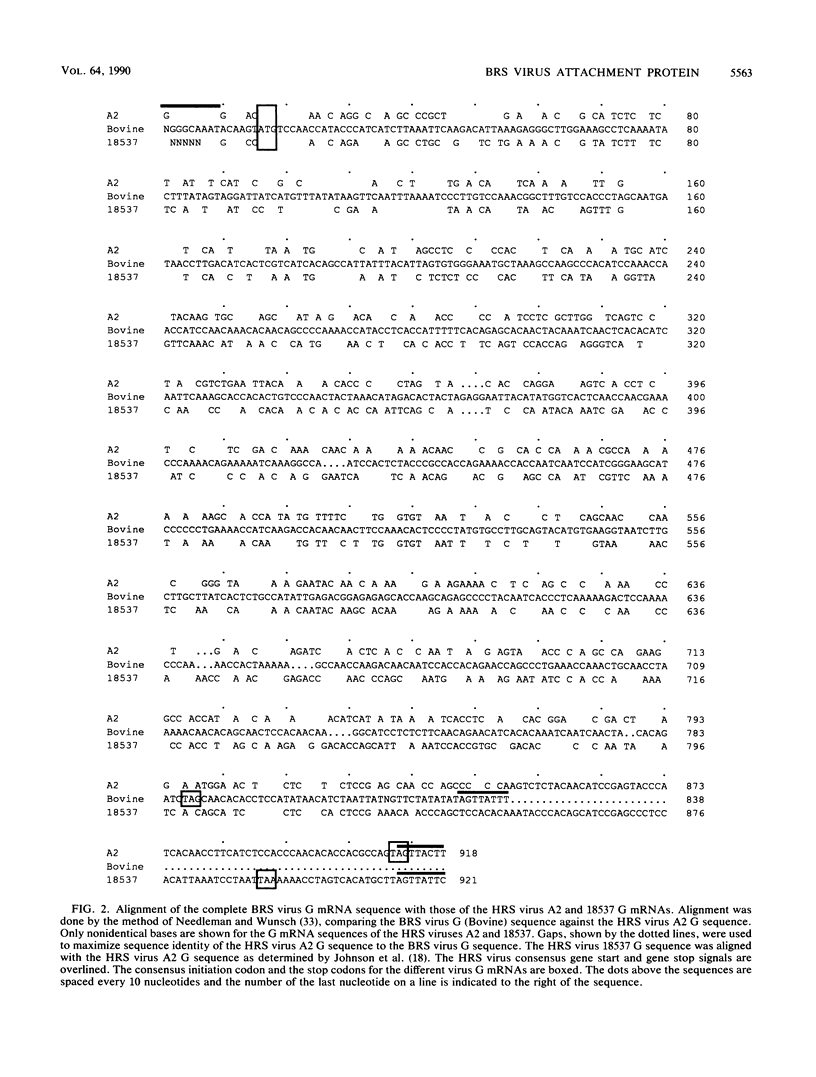
Abstract
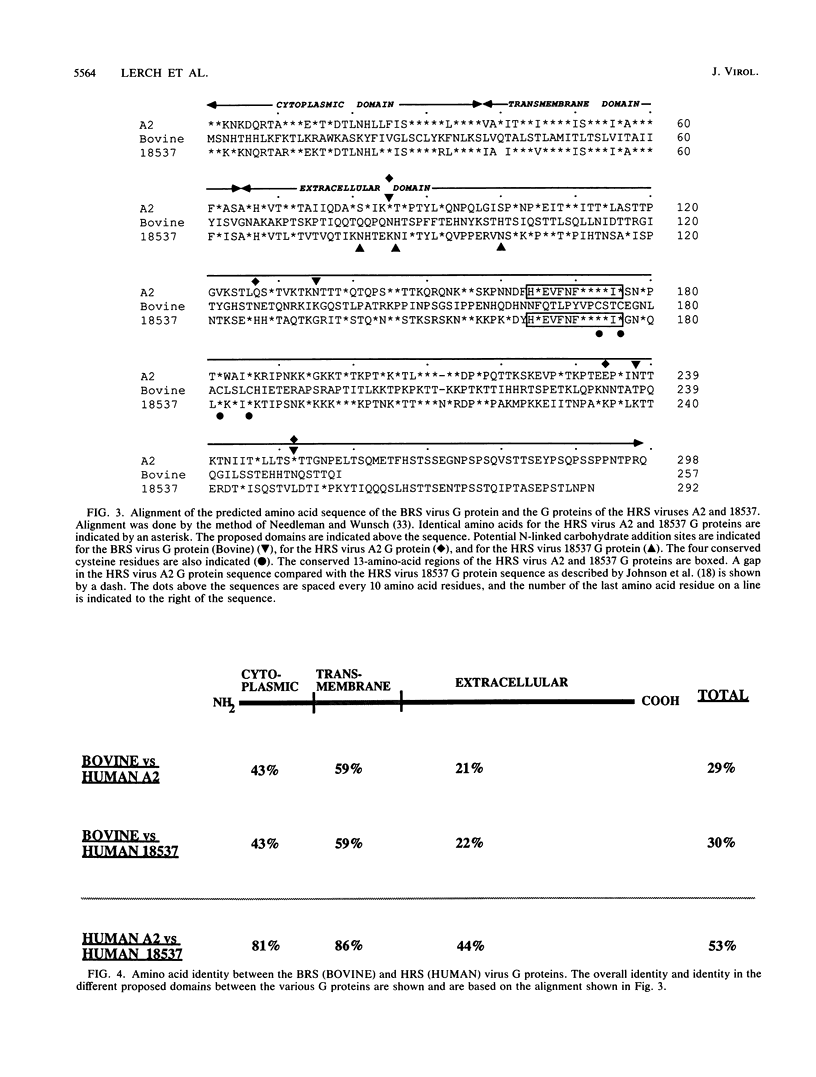
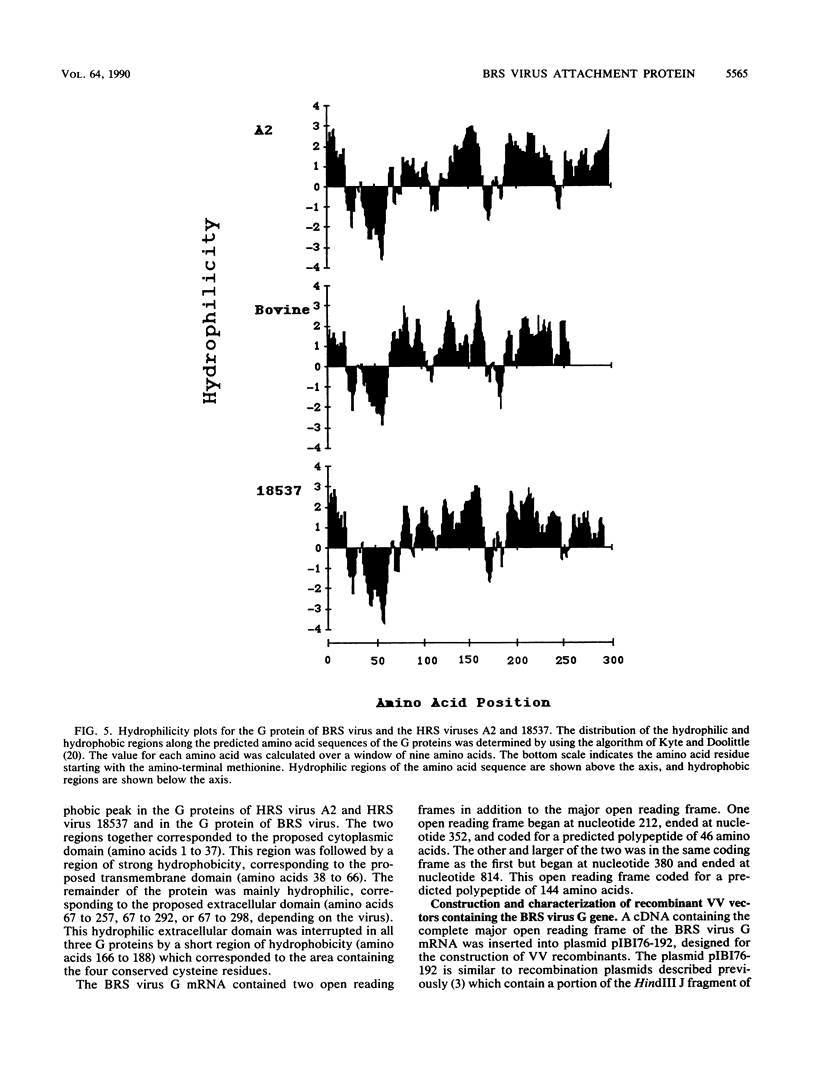
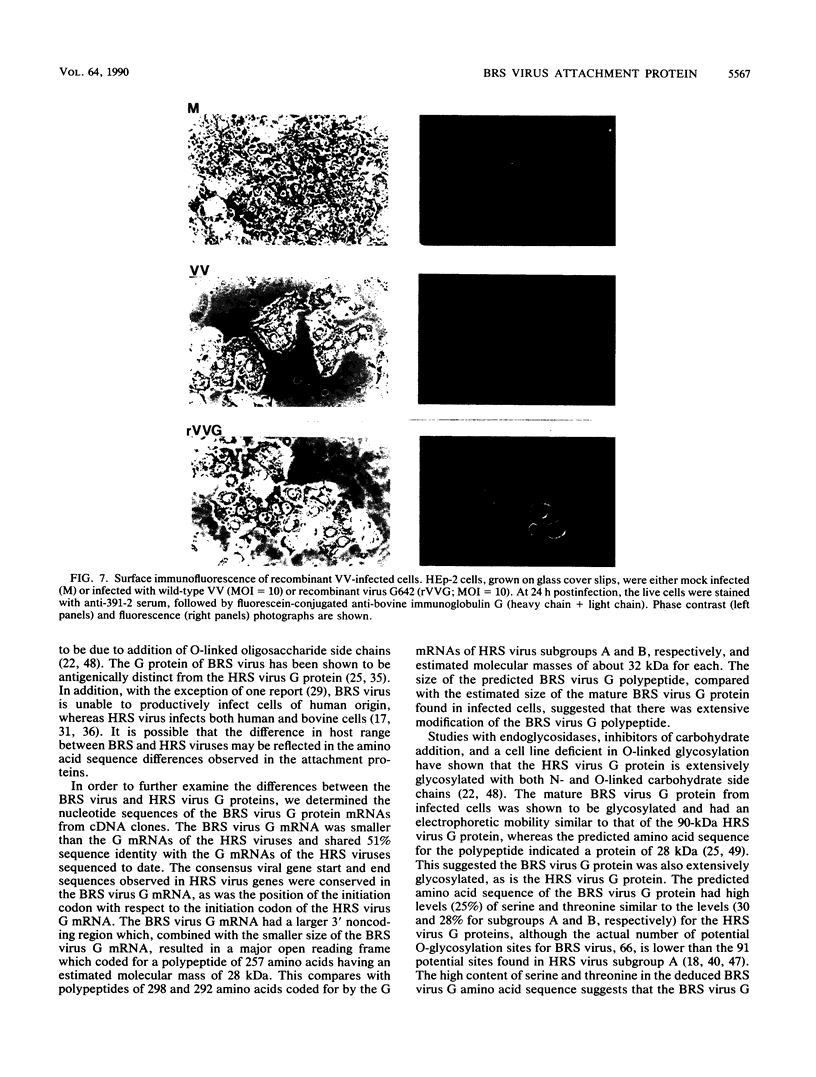
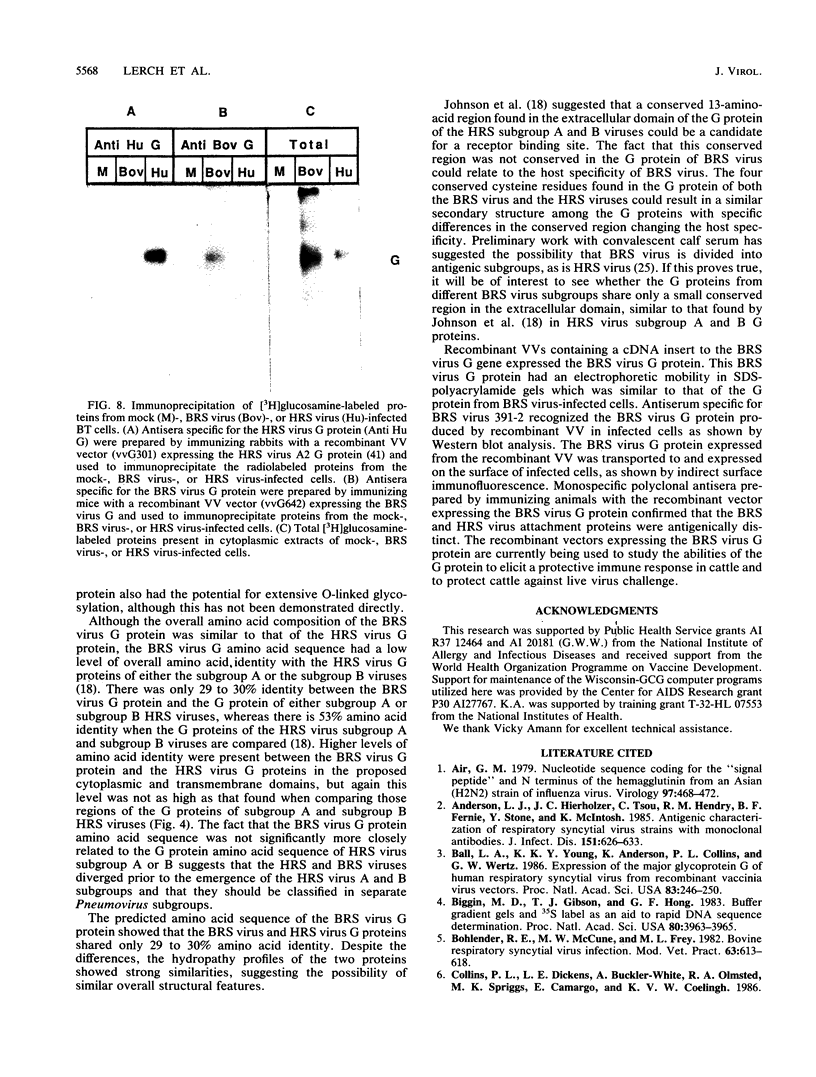
Bovine respiratory syncytial (BRS) virus causes a severe lower respiratory tract disease in calves similar to the disease in children caused by human respiratory syncytial (HRS) virus. While there is antigenic cross-reactivity among the other major viral structural proteins, the major glycoprotein, G, of BRS virus and that of HRS virus are antigenically distinct. The G glycoprotein has been implicated as the attachment protein for HRS virus. We have carried out a molecular comparison of the glycoprotein G of BRS virus with the HRS virus counterparts. cDNA clones corresponding to the BRS virus G glycoprotein mRNA were isolated and analyzed by dideoxynucleotide sequencing. The BRS virus G mRNA contained 838 nucleotides exclusive of poly(A) and had a major open reading frame coding for a polypeptide of 257 amino acid residues. The deduced amino acid sequence of the BRS virus G polypeptide showed only 29 to 30% amino acid identity with the G protein of either the subgroup A or B HRS virus. However, despite this low level of identity, there were strong similarities in the predicted hydropathy profiles of the BRS virus and HRS virus G proteins. A cDNA molecule containing the complete BRS virus G major open reading frame was inserted into the thymidine kinase gene of vaccinia virus by homologous recombination, and a recombinant virus containing the BRS virus G protein gene was isolated. This recombinant virus expressed the BRS virus G protein, as demonstrated by Western immunoblot analysis and immunofluorescence of infected cells. The BRS virus G protein expressed from the recombinant vector was transported to and expressed on the surface of infected cells. Antisera to the BRS virus G protein made by using the recombinant vector to immunize animals recognized the BRS virus attachment protein but not the HRS virus G protein and vice versa, confirming the lack of antigenic cross-reactivity between the BRS and HRS virus attachment proteins. On the basis of the data presented here, we conclude that BRS virus should be classified within the genus Pneumovirus in a group separate from HRS virus and that it is no more closely related to HRS virus subgroup A than it is to HRS virus subgroup B.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Air G. M. Nucleotide sequence coding for the "signal peptide" and N terminus of the hemagglutinin from an asian (H2N2) strain of influenza virus. Virology. 1979 Sep;97(2):468–472. doi: 10.1016/0042-6822(79)90358-1. [DOI] [PubMed] [Google Scholar]
- Anderson L. J., Hierholzer J. C., Tsou C., Hendry R. M., Fernie B. F., Stone Y., McIntosh K. Antigenic characterization of respiratory syncytial virus strains with monoclonal antibodies. J Infect Dis. 1985 Apr;151(4):626–633. doi: 10.1093/infdis/151.4.626. [DOI] [PubMed] [Google Scholar]
- Ball L. A., Young K. K., Anderson K., Collins P. L., Wertz G. W. Expression of the major glycoprotein G of human respiratory syncytial virus from recombinant vaccinia virus vectors. Proc Natl Acad Sci U S A. 1986 Jan;83(2):246–250. doi: 10.1073/pnas.83.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Dickens L. E., Buckler-White A., Olmsted R. A., Spriggs M. K., Camargo E., Coelingh K. V. Nucleotide sequences for the gene junctions of human respiratory syncytial virus reveal distinctive features of intergenic structure and gene order. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4594–4598. doi: 10.1073/pnas.83.13.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovi E. J. Analysis of proteins synthesized in respiratory syncytial virus-infected cells. J Virol. 1982 May;42(2):372–378. doi: 10.1128/jvi.42.2.372-378.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito J., Condit R., Obijeski J. The preparation of orthopoxvirus DNA. J Virol Methods. 1981 Feb;2(3):175–179. doi: 10.1016/0166-0934(81)90036-7. [DOI] [PubMed] [Google Scholar]
- Gruber C., Levine S. Respiratory syncytial virus polypeptides. III. The envelope-associated proteins. J Gen Virol. 1983 Apr;64(Pt 4):825–832. doi: 10.1099/0022-1317-64-4-825. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hruby D. E., Ball L. A. Control of expression of the vaccinia virus thymidine kinase gene. J Virol. 1981 Nov;40(2):456–464. doi: 10.1128/jvi.40.2.456-464.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. T., Collins P. L., Wertz G. W. Characterization of the 10 proteins of human respiratory syncytial virus: identification of a fourth envelope-associated protein. Virus Res. 1985 Mar;2(2):157–173. doi: 10.1016/0168-1702(85)90246-1. [DOI] [PubMed] [Google Scholar]
- Jacobs J. W., Edington N. Experimental infection of calves with respiratory syncytial virus. Res Vet Sci. 1975 May;18(3):299–306. [PubMed] [Google Scholar]
- Johnson P. R., Spriggs M. K., Olmsted R. A., Collins P. L. The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: extensive sequence divergence between antigenically related proteins. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5625–5629. doi: 10.1073/pnas.84.16.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambert D. M., Pons M. W. Respiratory syncytial virus glycoproteins. Virology. 1983 Oct 15;130(1):204–214. doi: 10.1016/0042-6822(83)90128-9. [DOI] [PubMed] [Google Scholar]
- Lambert D. M. Role of oligosaccharides in the structure and function of respiratory syncytial virus glycoproteins. Virology. 1988 Jun;164(2):458–466. doi: 10.1016/0042-6822(88)90560-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi S., Etkin S. Carcinogen-mediated induction of SV40 DNA synthesis in SV40 transformed Chinese hamster embryo cells. Carcinogenesis. 1981;2(5):417–423. doi: 10.1093/carcin/2.5.417. [DOI] [PubMed] [Google Scholar]
- Lerch R. A., Stott E. J., Wertz G. W. Characterization of bovine respiratory syncytial virus proteins and mRNAs and generation of cDNA clones to the viral mRNAs. J Virol. 1989 Feb;63(2):833–840. doi: 10.1128/jvi.63.2.833-840.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S., Klaiber-Franco R., Paradiso P. R. Demonstration that glycoprotein G is the attachment protein of respiratory syncytial virus. J Gen Virol. 1987 Sep;68(Pt 9):2521–2524. doi: 10.1099/0022-1317-68-9-2521. [DOI] [PubMed] [Google Scholar]
- Lim H. M., Pène J. J. Optimal conditions for supercoil DNA sequencing with the Escherichia coli DNA polymerase I large fragment. Gene Anal Tech. 1988 Mar-Apr;5(2):32–39. doi: 10.1016/0735-0651(88)90024-6. [DOI] [PubMed] [Google Scholar]
- Matumoto M., Inaba Y., Kurogi H., Sato K., Omori T. Bovine respiratory syncytial virus: host range in laboratory animals and cell cultures. Arch Gesamte Virusforsch. 1974;44(3):280–290. doi: 10.1007/BF01240616. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mohanty S. B., Lillie M. G., Ingling A. L. Effect of serum and nasal neutralizing antibodies on bovine respiratory syncytial virus infection in calves. J Infect Dis. 1976 Oct;134(4):409–413. doi: 10.1093/infdis/134.4.409. [DOI] [PubMed] [Google Scholar]
- Mufson M. A., Orvell C., Rafnar B., Norrby E. Two distinct subtypes of human respiratory syncytial virus. J Gen Virol. 1985 Oct;66(Pt 10):2111–2124. doi: 10.1099/0022-1317-66-10-2111. [DOI] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Olmsted R. A., Murphy B. R., Lawrence L. A., Elango N., Moss B., Collins P. L. Processing, surface expression, and immunogenicity of carboxy-terminally truncated mutants of G protein of human respiratory syncytial virus. J Virol. 1989 Jan;63(1):411–420. doi: 10.1128/jvi.63.1.411-420.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvell C., Norrby E., Mufson M. A. Preparation and characterization of monoclonal antibodies directed against five structural components of human respiratory syncytial virus subgroup B. J Gen Virol. 1987 Dec;68(Pt 12):3125–3135. doi: 10.1099/0022-1317-68-12-3125. [DOI] [PubMed] [Google Scholar]
- Paccaud M. F., Jacquier C. A respiratory syncytial virus of bovine origin. Arch Gesamte Virusforsch. 1970;30(4):327–342. doi: 10.1007/BF01258363. [DOI] [PubMed] [Google Scholar]
- Peeples M., Levine S. Respiratory syncytial virus polypeptides: their location in the virion. Virology. 1979 May;95(1):137–145. doi: 10.1016/0042-6822(79)90408-2. [DOI] [PubMed] [Google Scholar]
- Richman A. V., Pedreira F. A., Tauraso N. M. Attempts to demonstrate hemagglutination and hemadsorption by respiratory syncytial virus. Appl Microbiol. 1971 Jun;21(6):1099–1100. doi: 10.1128/am.21.6.1099-1100.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Satake M., Coligan J. E., Elango N., Norrby E., Venkatesan S. Respiratory syncytial virus envelope glycoprotein (G) has a novel structure. Nucleic Acids Res. 1985 Nov 11;13(21):7795–7812. doi: 10.1093/nar/13.21.7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott E. J., Ball L. A., Young K. K., Furze J., Wertz G. W. Human respiratory syncytial virus glycoprotein G expressed from a recombinant vaccinia virus vector protects mice against live-virus challenge. J Virol. 1986 Nov;60(2):607–613. doi: 10.1128/jvi.60.2.607-613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott E. J., Taylor G. Respiratory syncytial virus. Brief review. Arch Virol. 1985;84(1-2):1–52. doi: 10.1007/BF01310552. [DOI] [PubMed] [Google Scholar]
- Stott E. J., Thomas L. H., Collins A. P., Crouch S., Jebbett J., Smith G. S., Luther P. D., Caswell R. A survey of virus infections of the respiratory tract of cattle and their association with disease. J Hyg (Lond) 1980 Oct;85(2):257–270. doi: 10.1017/s0022172400063294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijaya S., Elango N., Zavala F., Moss B. Transport to the cell surface of a peptide sequence attached to the truncated C terminus of an N-terminally anchored integral membrane protein. Mol Cell Biol. 1988 Apr;8(4):1709–1714. doi: 10.1128/mcb.8.4.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir J. P., Moss B. Nucleotide sequence of the vaccinia virus thymidine kinase gene and the nature of spontaneous frameshift mutations. J Virol. 1983 May;46(2):530–537. doi: 10.1128/jvi.46.2.530-537.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz G. W., Collins P. L., Huang Y., Gruber C., Levine S., Ball L. A. Nucleotide sequence of the G protein gene of human respiratory syncytial virus reveals an unusual type of viral membrane protein. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4075–4079. doi: 10.1073/pnas.82.12.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz G. W., Krieger M., Ball L. A. Structure and cell surface maturation of the attachment glycoprotein of human respiratory syncytial virus in a cell line deficient in O glycosylation. J Virol. 1989 Nov;63(11):4767–4776. doi: 10.1128/jvi.63.11.4767-4776.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbrink F., Kimman T. G., Brinkhof J. M. Analysis of the antibody response to bovine respiratory syncytial virus proteins in calves. J Gen Virol. 1989 Mar;70(Pt 3):591–601. doi: 10.1099/0022-1317-70-3-591. [DOI] [PubMed] [Google Scholar]