Abstract
The tumour suppressor p53, which accumulates in response to DNA damage and induces cell-cycle arrest and apoptosis, has a key function in the maintenance of genome integrity. Under normal conditions, the antiproliferative effects of p53 are inhibited by MDM2, a ubiquitin ligase that promotes p53 ubiquitination and degradation. MDM2 is also self-ubiquitinated and degraded. Here, we show that the tumour suppressor RASSF1A regulates G1–S cell-cycle progression in a p53-dependent manner by promoting MDM2 self-ubiquitination and preventing p53 degradation. Importantly, RASSF1A associates with MDM2 and death-domain-associated protein (DAXX) in the nucleus, thereby disrupting the interactions between MDM2, DAXX, and the deubiquitinase, HAUSP, and enhancing the self-ubiquitin ligase activity of MDM2. Moreover, RASSF1A partially contributes to p53-dependent checkpoint activation at early time points in response to DNA damage. These findings reveal a new and important function for RASSF1A in regulating the p53–MDM2 pathway.
Keywords: DAXX, MDM2, p53, RASSF1A
Introduction
The tumour suppressor p53 has an important function in protecting the integrity of the genome. In response to various types of stress, p53 is stabilized and activated, resulting in cell-cycle arrest, apoptosis, or senescence (Vogelstein et al, 2000; Michael and Oren, 2003). The stability of p53 is mainly regulated by the RING domain-containing E3 ubiquitin ligase, MDM2 (Haupt et al, 1997; Kubbutat et al, 1997), and in normal unstressed cells p53 has a short half-life. The MDM2 gene is also transactivated by p53; thus, MDM2 and p53 together form a negative feedback loop (Prives, 1998). Several pathways leading to inhibition of MDM2 action have been identified, including the phosphorylation of MDM2 by DNA-damage-induced kinases (Khosravi et al, 1999; Maya et al, 2001; Stommel and Wahl, 2004) and the interaction of MDM2 with other proteins (Brooks and Gu, 2006; Toledo and Wahl, 2006), including ARF, which disrupts MDM2 regulation of p53 by inhibiting MDM2 ubiquitin ligase activity (Kamijo et al, 1998; Pomerantz et al, 1998; Zhang et al, 1998; Sherr and Weber, 2000). Ribosomal proteins, such as L11, L23, and L5, also inhibit MDM2 activity and disrupt p53 regulation (Lohrum et al, 2003; Zhang et al, 2003; Dai and Lu, 2004; Dai et al, 2004; Jin et al, 2004). Protein YY1 and the oncoprotein gankyrin have also been suggested as potentiating cofactors for MDM2, facilitating MDM2–p53 interaction and p53 ubiquitination (Sui et al, 2004; Higashitsuji et al, 2005). Interestingly, MDM2 is an unstable protein that is ubiquitinated in an autocatalytic manner (Fang et al, 2000; Stommel and Wahl, 2004). MDM2 distinguishes between self-ubiquitination and target p53 ubiquitination, and thus mechanisms that regulate these respective activities are important for regulation of p53 activity.
Recently, it has been demonstrated that death-domain-associated protein (DAXX), together with the deubiquitinating enzyme, HAUSP, can prevent MDM2 self-ubiquitination and enhance the activity of MDM2 towards p53, thereby promoting p53 degradation (Tang et al, 2006). DAXX is also an apoptosis regulatory protein that links the death receptor Fas to c-Jun NH2-terminal kinase (JNK) (Yang et al, 1997). However, Daxx-null mice die at early embryonic stages because of extensive cell death, indicating an additional function for Daxx in regulating cell survival during development (Michaelson et al, 1999). HAUSP (also known as USP7) interacts with p53, leading to p53 deubiquitination and stabilization (Li et al, 2002). However, the ablation of HAUSP expression results in an increase in p53 and p53-dependent G1 arrest, as well as a marked reduction in MDM2, indicating that HAUSP also has a key function in regulating MDM2 stability (Cummins et al, 2004; Li et al, 2004; Meulmeester et al, 2005). Furthermore, the MDM2-related protein, MDMX, also regulates MDM2 activity, with implications for MDM2-dependent regulation of p53 (Parant et al, 2001). MDMX does not directly mediate the ubiquitination and destruction of p53, but rather appears to be required for MDM2 stability and MDM2-mediated p53 degradation. However, an excess of MDMX stabilizes p53 (Linares et al, 2003; Marine and Jochemsen, 2004).
RASSF1A is a tumour suppressor gene that is inactivated in many human cancers (Dammann et al, 2000). Mice with homozygous deletions of RASSF1A are prone to tumour development (Tommasi et al, 2005; van der Weyden et al, 2005). Both RASSF1A and RASSF1C contain C-terminal SARAH protein–protein interaction and RA domains, but differ in their N-terminal sequences. Interestingly, only RASSF1A, which serves multiple functions in a diverse series of critical cellular processes by interacting with other proteins, has been shown to function as a tumour suppressor (Agathanggelou et al, 2005; Donninger et al, 2007). RASSF1A regulates mitotic progression by influencing microtubule dynamics and the activity of APC–Cdc20 (Liu et al, 2003; Rong et al, 2004; Song et al, 2004; Vos et al, 2004). RASSF1A also binds the p120 E4F transcription factor, which forms a complex with both the Rb and p53 tumour suppressors (Fenton et al, 2004). In addition, RASSF1A induces a G1 arrest through inhibition of cyclin D1, thereby suggesting a function for RASSF1A in the G1–S transition (Shivakumar et al, 2002). In fact, RASSF1A undergoes ubiquitin-mediated degradation by Skp2 at the G1–S transition (Song et al, 2008). RASSF1A has also been implicated in the regulation of apoptosis, acting to link death receptor signaling to the apoptosis machinery and serving as a part of the mammalian Hippo complex, which is involved in apoptosis and cell proliferation (Baksh et al, 2005; Oh et al, 2006; Guo et al, 2007). Moreover, RASSF1A induces apoptosis by promoting the dissociation of MST2 from Raf and facilitating MST2 interaction with LATS1 after Fas treatment (Matallanas et al, 2007). RASSF1A and RASSF1C bind to DAXX, and RASSF1C is released from DAXX after DNA damage, subsequently translocating to cytoplasmic microtubules and activating SAPK/JNK (Kitagawa et al, 2006). However, the full spectrum of RASSF1A functions, particularly in DNA-damaged cells, remains unclear. Here, we show that RASSF1A disrupts interactions among MDM2, DAXX, and HAUSP, thus enhancing MDM2 self-ubiquitination and stabilizing p53 during an early stage in response to DNA damage.
Results
Overexpression of RASSF1A induces a p53-dependent delay in G1–S cell-cycle progression
Several studies have suggested a growth-inhibitory function of RASSF1A at G1 and during mitosis (Shivakumar et al, 2002; Liu et al, 2003; Rong et al, 2004; Song et al, 2004) that is cell line context-dependent. Previously, we reported that ectopic expression of RASSF1A induced a delay in G1–S cell-cycle progression in cell lines that express wild-type p53 (U2OS, A549, and MCF7) but not in those (SaoS2, H1299, HeLa, and 293T) in which p53 is inactive (Song et al, 2008), suggesting that RASSF1A likely modulates G1–S progression in a p53-dependent manner. To exclude the possibility that this difference in the effect of RASSF1A might reflect something other than p53 status, we first examined parental p53 wild-type HCT116 cells and isogenic HCT116 cells in which both p53 alleles had been subjected to targeted deletion. Exogenous expression of RASSF1A in p53+/+ HCT116 cells increased the proportion of cells in G1 from 29.84 to 43.48%, whereas RASSF1A expression in p53−/− HCT116 cells increased the fraction of cells in G2–M (Figure 1A). Exogenous expression of RASSF1A with restoration of p53 expression in p53−/− cells also slightly increased the number of cells at G1. Notably, RASSF1A also induced the accumulation of both p53 and p21 in p53 wild-type cells, but not in p53−/− HCT116 cells (Figure 1B). By contrast, RASSF1C failed to increase the number of cells at G1 or the abundance or activity of p53 (Supplementary Figure S1A and B). In addition, the level of 5-bromo-2-deoxyuridine (BrdU) incorporation into DNA was reduced in U2OS cells expressing hemagglutinin (HA)-RASSF1A (Figure 1C). To determine whether the apparent increase in p53 levels induced by RASSF1A was associated with an increase in p53 activity, we examined the effects of RASSF1A on p53 transcriptional activity in U2OS cells transfected with a p53-responsive promoter–reporter construct. RASSF1A, but not RASSF1C, caused a marked increase in both the abundance and activity of wild-type p53 (Figure 1D and Supplementary Figure S1C). Collectively, these results suggest a functional link between RASSF1A and p53 in cell-cycle control.
Figure 1.
Overexpression of RASSF1A delays G1–S progression in a p53-dependent manner. (A) HCT116 cells (p53+/+, p53−/− and p53−/− complemented with HA–p53) were transfected with pcDNA-CD4 and HA–RASSF1A. The cell-cycle profile of CD4-positive cells was determined 36 h after transfection. Data are expressed as means±standard errors of values from three independent experiments. (B) Cells in (A) were analysed by immunoblotting for the indicated proteins. Asterisk indicates nonspecific bands. (C) U2OS cells incubated for the indicated times after transfection with HA–RASSF1A were exposed during the final 1 h to 10 μM BrdU and then analysed by flow cytometry to determine the percentage of BrdU-positive cells. Data are expressed as means±standard errors of values from three independent experiments. (D) U2OS cells were co-transfected with pGL3-p53-responsive reporter and pcDNA3-β-Gal reporter constructs and the indicated combinations of pcDNA3-p53 (wt), pcDNA3-p53-R248W (mt) and HA–RASSF1A. Cell lysates were subjected to immunoblot and luciferase assays. Luciferase activity was normalized to β-galactosidase activity and expressed relative to that in cells transfected with an empty vector. Data are expressed as means±standard errors of values from three independent experiments.
RASSF1A promotes the stability and activity of p53 by antagonizing MDM2
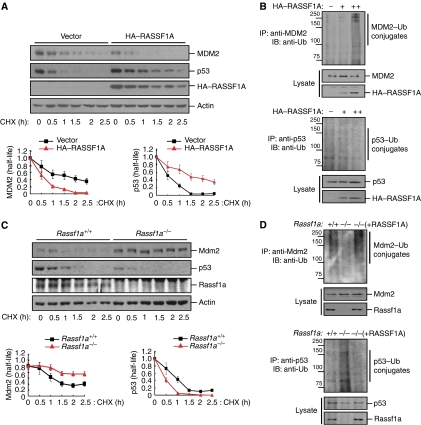
The role of p53 in cell-cycle control is modulated by mechanisms that affect p53 stability and activity. To determine whether RASSF1A affects the stability of p53 directly or through MDM2, we first determined whether RASSF1A affects MDM2 ubiquitin ligase activity by measuring the stability of MDM2 and p53 in U2OS cells overexpressing RASSF1A. Interestingly, overexpression of RASSF1A decreased MDM2 half-life and increased the levels of ubiquitinated MDM2 (Figure 2A and B). Consequently, the half-life of endogenous p53 was prolonged and ubiquitination of p53 was inhibited by RASSF1A but not RASSF1C (Figure 2A and B and Supplementary Figure S2). We next examined the half-lives of endogenous Mdm2 and p53 in Rassf1a wild-type and null mouse embryo fibroblasts (MEFs). Compared with wild-type MEFs, the half-life of p53 was reduced in Rassf1a−/− MEFs in association with an increase in the half-life of Mdm2 (Figure 2C). Consistent with this observation, Mdm2 ubiquitination was reduced in Rassf1a−/− MEFs, whereas ubiquitination of p53 was increased; these changes were reversed in Rassf1a−/− MEFs complemented with RASSF1A (Figure 2D). Moreover, exogenous expression of RASSF1A stabilized ectopically expressed p53 in Mdm2+/+p53−/− MEFs compared with vector-only controls (Supplementary Figure S3A, left). By contrast, the stability of ectopic p53 in Mdm2−/−p53−/− MEFs was prolonged with or without exogenous expression of RASSF1A (Supplementary Figure S3A, right), indicating that excess RASSF1A likely stabilized p53, even in the presence of Mdm2.
Figure 2.
RASSF1A regulates the half-life and stability of MDM2 and p53. (A) U2OS cells transfected with HA–RASSF1A were incubated for the indicated times with cycloheximide (CHX; 50 μg/ml) and analysed by immunoblotting. Relative levels of MDM2 and p53 were quantified and normalized to actin. Error bars indicate standard errors. (B) U2OS cells transfected with increasing amounts of HA–RASSF1A were treated with MG132 for 6 h and then cell lysates were immunoprecipitated with anti-MDM2 or anti-p53 antibodies. The resulting immunoprecipitates (IPs) were analysed by immunoblotting (IB) using an anti-ubiquitin antibody. Molecular weights are in kDa. (C) Rassf1a+/+ or Rassf1a−/− MEFs were incubated with cycloheximide (50 μg/ml) for the indicated times and analysed by immunoblotting. Relative levels of Mdm2 and p53 were quantified and normalized to actin. Error bars indicate standard errors. (D) Rassf1a+/+ or Rassf1a−/− MEFs were treated with MG132 for 6 h and cell lysates were analysed for Mdm2 and p53 ubiquitination by immunoblotting. Molecular weights are in kDa.
Finally, given that MDM2 targets p53 for degradation and that MDM2 overexpression abolishes p53-mediated G1 arrest (Zhang et al, 1998), we examined whether RASSF1A might functionally antagonize MDM2 activity. Ectopic expression of p53 induced G1 arrest in U2OS cells, an effect that was prevented by overexpression of MDM2 (Figure 3A, left). However, co-transfection of cells with expression plasmids for RASSF1A, p53, and MDM2 restored the p53-induced G1 arrest. This effect of RASSF1A was accompanied by restoration of the levels of p53 and p21 to those in cells transfected with only the p53 expression construct, and by a marked reduction in the abundance of MDM2 (Figure 3A, right). By contrast, RASSF1C failed to restore p53-induced G1 arrest and the levels of p53 and p21 in the presence of MDM2 (Figure 3B). The effect of RASSF1A on p53 abundance was also evidenced by nuclear accumulation of p53 in the presence of ectopically expressed MDM2 (data not shown). We next examined the levels of ubiquitinated p53 and MDM2 in Mdm2−/−p53−/− MEFs exogenously expressing p53, MDM2, and His–ubiquitin, and either RASSF1A or RASSF1C (Figure 3C). Consistent with our previous observations, RASSF1A, but not RASSF1C, increased MDM2 ubiquitination and reduced MDM2-mediated p53 ubiquitination. Collectively, these data indicate that RASSF1A promotes the stability and activity of p53, most likely by antagonizing MDM2.
Figure 3.
RASSF1A regulates p53 stability by inhibiting MDM2-mediated p53 degradation. (A, B) U2OS cells were co-transfected with pcDNA-CD4 and expression vectors for HA–p53, pCMV-MDM2, HA–RASSF1A, or HA–RASSF1C as indicated. The cell-cycle profile of CD4-positive cells was analysed 36 h after transfection (left) and the cell lysates were analysed by immunoblotting (right). Asterisk indicates nonspecific bands. (C) Mdm2−/−p53−/− MEFs were co-transfected with MDM2, HA–p53, and His6-tagged ubiquitin, with or without co-transfection of HA–RASSF1A or HA–RASSF1C. Cells were treated with MG132 for 6 h prior to harvesting. The His6-purified fraction was analysed for ubiquitinated MDM2 and p53. Molecular weights are in kDa.
RASSF1A associates with both MDM2 and DAXX
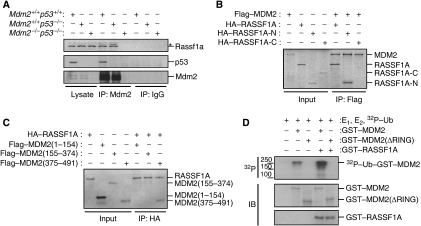
To investigate the mechanism by which RASSF1A regulates MDM2-mediated p53 degradation, we first tested whether RASSF1A might bind to MDM2. A co-immunoprecipitation assay detected interaction between endogenous RASSF1A and MDM2 in several cell lines even in the absence of p53, indicating that the interaction is independent of p53 (Figure 4A and data not shown). In vitro binding experiments also revealed that RASSF1A interacted with MDM2 (Figure 4B and C), but did not interact directly with p53 (Supplementary Figure S3B). Although RASSF1A did not associate with ectopically expressed p53 in Mdm2−/−p53−/− MEFs, RASSF1A formed a complex with p53 in the presence of ectopic MDM2 (Supplementary Figure S3C). Thus, RASSF1A does not interact directly with p53 but is able to form a complex with MDM2 and p53 in vivo. To determine whether endogenous RASSF1A colocalizes with MDM2, we used an immunocytological approach. Although RASSF1 is well known to localize to microtubules, RASSF1A localization to the nucleus may be underestimated. Under the conditions used (see Materials and methods), immunostaining clearly showed that endogenous RASSF1A was localized to cytoplasmic microtubules and colocalized with MDM2 in the nucleus (Supplementary Figure S4A). These results were also confirmed by western blotting and co-immunoprecipitation assays, which detected a significant amount of endogenous RASSF1A in the nuclear fraction and demonstrated an interaction between nuclear RASSF1A and MDM2 (Supplementary Figure S4B).
Figure 4.
RASSF1A interacts with MDM2 in vivo and in vitro. (A) Lysates from p53+/+, p53−/− and Mdm2−/−p53−/− MEFs were immunoprecipitated with anti-Mdm2 antibodies and the resulting immunoprecipitates were analysed by immunoblotting. Asterisk indicates nonspecific bands. (B, C) The indicated fragments of RASSF1A and MDM2 were produced by in vitro translation in the presence of [35S]methionine. Fragments were mixed, immunoprecipitated with anti-Flag (B) or anti-HA (C) antibodies and analysed by SDS–PAGE and autoradiography. (D) Self-ubiquitylation of GST–MDM2 and GST–MDM2(ΔRING) with or without GST–RASSF1A was detected using 32P ubiquitin or immunoblot analysis (IB) with α-GST. Molecular weights are in kDa.
We next determined which functional domains of MDM2 and RASSF1A were required for their interaction. An in vitro binding assay using deletion mutants of both proteins revealed that the N-terminal region (residues 1–119, RASSF1A-N) of RASSF1A (Figure 4B), but not the C-terminal region (residues 120–340, RASSF1A-C), associated with the C-terminal region (residues 375–491) of MDM2, which contains the RING domain. No specific binding was detected with the MDM2 N-terminal region (residues 1–154), containing the p53-interacting domain, or the central region (residues 155–374), containing the acidic domain (Figure 4C). Taken together, these in vitro binding data and the failure of RASSF1C to enhance MDM2 ubiquitination activity (Figure 3C) indicate that the N-terminal region of RASSF1A is likely necessary for MDM2 to physically interact with and stabilize p53. As no major difference of RASSF1A level was detected with MDM2 overexpression in Mdm2−/−p53−/− MEFs (Supplementary Figure S5A), RASSF1A may not be a substrate for MDM2. In addition, neither the N-terminal region alone nor the C-terminal part of RASSF1A was sufficient to affect the MDM2 and p53 stability, suggesting that full length of RASSF1A is required for the regulation of the MDM2 and p53 stability (Supplementary Figure S5B and C). Finally, an in vitro gel-based assay using 32P-labelled ubiquitin revealed that recombinant RASSF1A increased the self-ubiquitination of wild-type MDM2 but not that of an MDM2 mutant (MDM2(ΔRING)) lacking the RING domain (Figure 4D). These results suggest that RASSF1A regulates the intrinsic self-ubiquitination ligase activity of MDM2 in vitro.
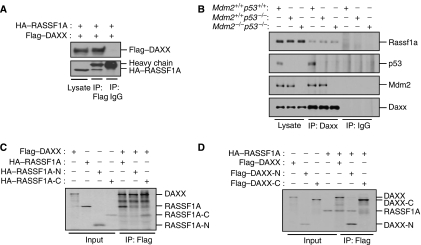
It has recently been shown that DAXX binds to MDM2 and is required for MDM2 activity towards p53 (Tang et al, 2006). DAXX also associates with RASSF1A and RASSF1C in vivo (Kitagawa et al, 2006). To determine whether RASSF1A might regulate MDM2 stability by interacting with DAXX, we employed a co-immunoprecipitation assay and found that both exogenous and endogenous RASSF1A interacted with DAXX (Figure 5A and B). This interaction of RASSF1A with Daxx was independent of Mdm2 and p53 (Figure 5B). Immunofluorescence analysis also revealed that RASSF1A and DAXX colocalized in the nucleus of Triton X-100-permeabilized, fixed cells (Supplementary Figure S4C). Consistent with previous findings (Kitagawa et al, 2006), we found that this association was mediated by an interaction between the RASSF1A C-terminal region (residues 120–340, RASSF1A-C) and the DAXX N-terminal region (residues 1–240, DAXX-N) containing the MDM2- and HAUSP-interacting domains (Figure 5C and D). Collectively, these data indicate that RASSF1A associates with both MDM2 and DAXX in the nucleus.
Figure 5.
RASSF1A interacts with DAXX in vivo and in vitro. (A) 293T cells transfected with Flag–DAXX and HA–RASSF1A expression plasmids were immunoprecipitated (IP) with anti-Flag or immunoglobulin G (IgG) antibodies and the resulting precipitates and cell lysates were subjected to immunoblot analysis. (B) Lysates from p53+/+, p53−/− and Mdm2−/−p53−/− MEFs were immunoprecipitated with an anti-Daxx antibody, and the resulting immunoprecipitates (IPs) were analysed by immunoblotting. The indicated fragments of RASSF1A (C) or Daxx (D) were produced by in vitro translation in the presence of [35S]methionine. Fragments were mixed, immunoprecipitated (IP) with an anti-Flag antibody and analysed by SDS–PAGE and autoradiography.
RASSF1A increases MDM2 self-ubiquitination by disrupting MDM2–DAXX–HAUSP interactions
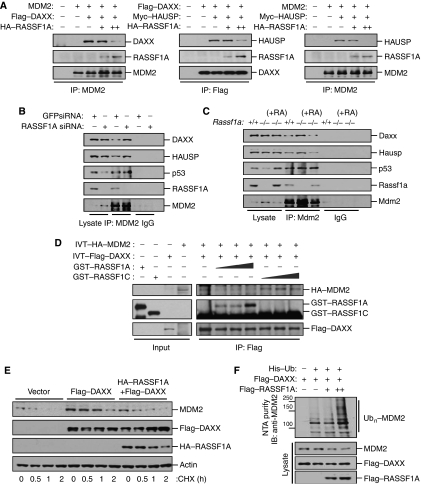
DAXX-N is able to associate with MDM2 or HAUSP as well as with RASSF1A, and the effects of RASSF1A on MDM2 stability are opposite to those of DAXX and HAUSP (Tang et al, 2006, and this study). These observations suggest that RASSF1A might affect MDM2–DAXX–HAUSP interactions, thus preventing DAXX and HAUSP from stabilizing MDM2. Co-transfection and co-immunoprecipitation assays showed that RASSF1A interfered with the interaction of MDM2 and the deubiquitinating enzyme, HAUSP, and also inhibited the interactions between MDM2 and DAXX or HAUSP, and DAXX and HAUSP (Figure 6A and Supplementary Figure S6). By contrast, the association of MDM2 with DAXX or HAUSP was increased in RASSF1A-depleted U2OS cells (Figure 6B). Restoration of RASSF1A expression in Rassf1a−/− MEFs reversed the observed increases in Mdm2–Hausp and Mdm2–Daxx associations (Figure 6C). Taken together, these data suggest that RASSF1A prevents the association of DAXX–HAUSP with MDM2. Because DAXX also binds to RASSF1C (Figure 5C and Kitagawa et al, 2006), we sought to determine whether RASSF1C was also able to inhibit DAXX–HAUSP–MDM2 interactions. Co-transfection and co-immunoprecipitation assays suggested that RASSF1C did not significantly reduce DAXX–MDM2 interactions in vivo (Supplementary Figure S7A). Most importantly, the interaction of the deubiquitinating enzyme HAUSP with either DAXX or MDM2 was not affected by exogenous RASSF1C (Supplementary Figure S7B and C). An in vitro competitive binding assay revealed that RASSF1A, but not RASSF1C, interfered with MDM2–DAXX interactions (Figure 6D). These results suggest that the ability of RASSF1A to prevent MDM2–DAXX interactions requires both MDM2 binding to the N terminus of RASSF1A and DAXX binding to the C terminus of RASSF1A.
Figure 6.
RASSF1A increases MDM2 self-ubiquitination by disrupting MDM2–DAXX–HAUSP interactions. (A) Mdm2−/−p53−/− MEFs transfected with MDM2 and Flag–DAXX or Myc–HAUSP and increasing amounts of HA–RASSF1A were treated with MG132 for 6 h. Cell lysates were then immunoprecipitated (IP) with anti-MDM2 or anti-Flag antibodies, and the resulting precipitates were analysed by immunoblotting. (B) U2OS cells transfected with GFP siRNA or RASSF1A siRNA were immunoprecipitated (IP) with an anti-MDM2 antibody and the resulting precipitates were analysed by immunoblotting. (C) Rassf1a+/+, Rassf1a−/−, and Rassf1a−/− MEFs complemented with pcDNA-RASSF1A were immunoprecipitated (IP) with anti-Mdm2 and the resulting precipitates were analysed by immunoblotting. (D) GST–RASSF1A (70, 140, and 210 ng) or GST–RASSF1C (70, 140, and 210 ng) were added to a mixture of in vitro-translated (IVT) HA–MDM2 and Flag–DAXX and incubated for 2 h at room temperature, followed by immunoprecipitation (IP) with an anti-Flag antibody and immunoblotting. (E) U2OS cells transfected with Flag–DAXX with or without HA–RASSF1A were incubated for the indicated times with cycloheximide (50 μg/ml) and analysed by immunoblotting. (F) U2OS cells were transfected with a combination of Flag–DAXX and Flag–RASSF1A in the presence of a His6-tagged ubiquitin expression plasmid. Cells were treated with MG132 for 6 h prior to harvesting. The His6-purified fractions were analysed for ubiquitinated MDM2.
We next investigated the function of RASSF1A in DAXX- and HAUSP-mediated MDM2 stabilization and deubiquitination. Ectopic expression of DAXX or HAUSP in U2OS cells strongly stabilized endogenous MDM2. However, in cells that expressed RASSF1A in addition to DAXX or HAUSP, the stabilizing effect of DAXX or HAUSP on MDM2 was significantly reduced (Figure 6E and Supplementary Figure S8A). Consistent with this, exogenous RASSF1A enhanced MDM2 ubiquitination even in the presence of ectopically expressed DAXX or HAUSP (Figure 6F and Supplementary Figure S8B). Collectively, these data suggest that RASSF1A interferes with the interaction of MDM2 with HAUSP and the adaptor protein DAXX in the regulation of MDM2 stability, which in turn, might enhance the MDM2 self-ubiquitination.
RASSF1A contributes to p53 activation in response to DNA damage
Recent studies have shown that an early step in the accumulation of p53 in response to DNA damage is an associated increase in MDM2 self-ubiquitination, which is probably facilitated by disruption of MDM2–DAXX–HAUSP interactions (Stommel and Wahl, 2004; Tang et al, 2006). Consistent with this early involvement of MDM2 self-ubiquitination in DNA damage response, we found that treatment of several cell lines with the DNA-damaging agent adriamycin reduced levels of MDM2 at early time points (Figure 8 and data not shown). The effect of RASSF1A on MDM2 destabilization led us to investigate whether DNA damage might regulate the interactions between RASSF1A, MDM2, DAXX, and HAUSP. Treatment of U2OS cells with adriamycin abolished MDM2–DAXX–HAUSP interactions after 30 min and was accompanied by enhanced MDM2 self-ubiquitination (Figure 7A and B). Interestingly, both RASSF1A–MDM2 and RASSF1A–DAXX interactions appeared to be slightly increased at 60 min after adriamycin treatment. By contrast, the MDM2–DAXX–HAUSP complex remained intact after adriamycin treatment of both RASSF1A-depleted U2OS cells (Figure 7C, left) and Rassf1a−/− MEFs (Figure 7D, left). In agreement with this, adriamycin-induced MDM2 ubiquitination was significantly reduced in both RASSF1A-deficient U2OS cells (Figure 7C, right) and Rassf1a−/− MEFs (Figure 7D, right). Taken together, these data suggest that RASSF1A is involved in disrupting the MDM2–DAXX–HAUSP complex and promoting MDM2 self-ubiquitination upon DNA damage.
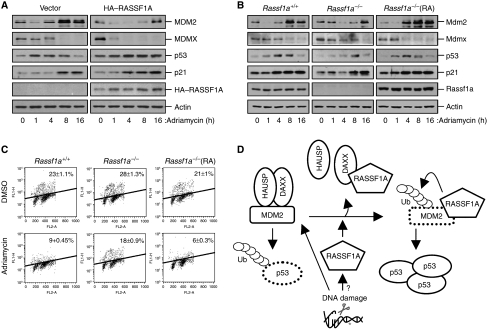
Figure 8.
RASSF1A contributes to the efficient activation of p53 at early time points after DNA damage. (A) MCF7 cells transfected with pcDNA-CD4 and HA–RASSF1A (or with the corresponding empty vectors) were treated with adriamycin (1 μM) for the indicated times. Cell lysates were analysed by immunoblotting for the indicated proteins. (B) Rassf1a+/+, Rassf1a−/− and Rassf1a−/− MEFs complemented with pcDNA-RASSF1A were treated with adriamycin (1 μM) for the indicated times, followed by immunoblot analysis of cell lysates. (C) Rassf1a+/+, Rassf1a−/− and Rassf1a−/− MEFs complemented with pcDNA-RASSF1A were treated with adriamycin (1 μM) for 12 h and with BrdU for 30 min prior to harvest. Cell-cycle profiles and fractions of S-phase (BrdU-positive) cells were analysed by flow cytometry. Data are expressed as means±s.e.m. of values from three independent experiments. (D) A model depicting the postulated RASSF1A-mediated regulation of MDM2 and p53 stability following DNA damage through disruption of MDM2–DAXX–HAUSP interactions.
Figure 7.
RASSF1A disrupts MDM2–DAXX–HAUSP interactions and is required for MDM2 self-ubiquitination upon DNA damage. (A, B) U2OS cells were treated with MG132 for 6 h and then with adriamycin (1 μM) for the indicated times. MDM2–DAXX–HAUSP interactions (A) were analysed by immunoprecipitation (IP) with IgG (lane 1) and MDM2 (lane 2–8), followed by immunoblotting for ubiquitinated MDM2 (B). Molecular weights are in kDa. (C, D) U2OS cells transfected with GFP siRNA or RASSF1A siRNA (C), or Rassf1a+/+, Rassf1a−/− and Rassf1a−/− MEFs complemented with pcDNA-RASSF1A (D), were treated with adriamycin (1 μM) for 30 min. Mdm2-–Daxx–Hausp interactions were analysed by immunoprecipitation (IP) with the indicated antibodies (left) followed by immunoblotting for ubiquitinated Mdm2 (right). Molecular weights are in kDa.
In response to DNA damage, activated p53 serves the important function of arresting the cell cycle in the G1 or G2 phase, a process in which the p53 target, p21, has a major function (Bunz et al, 1998). Given that RASSF1A mediates MDM2 destabilization and p53 stabilization, we examined whether RASSF1A might contribute to p53-dependent control of cell growth in response to DNA damage. Ectopic expression of RASSF1A in adriamycin-treated MCF7 cells, which do not express RASSF1A, induced G1 arrest and promoted accumulation of both p53 and p21 compared with controls, suggesting that RASSF1A contributes to p53 activation at an early stage in response to DNA damage (Figure 8A). Moreover, the transient reduction in Mdm2 abundance at early time points after adriamycin treatment was not apparent in Rassf1a−/− MEFs (Figure 8B). By contrast, restoration of RASSF1A expression in Rassf1a−/− MEFs, however, recovered the increases in p53 and p21 levels, and the early decrease in Mdm2 stability induced by adriamycin (Figure 8B). Rassf1a+/+ MEFs displayed reduced numbers of BrdU-labelled cells upon adriamycin treatment, indicating the cell-cycle arrest at G1–S. By contrast, the numbers of BrdU-labelled cells were not significantly decreased in Rassf1a−/− MEFs upon adriamycin treatment (Figure 8C). Restoration of RASSF1A expression in Rassf1a−/− MEFs, however, restored the cell-cycle arrest in response to adriamycin. We also found that induction of RASSF1A expression in stably transfected U2OS cells markedly potentiated both γ-irradiation (IR)-induced p53 and p21 accumulation and the IR-induced decrease in MDM2 levels (Supplementary Figure S9). Collectively, these data suggest that RASSF1A contributes to the efficient activation of p53 by promoting MDM2 self-ubiquitination in cell-cycle checkpoint control in response to DNA damage.
Discussion
The results described in this study demonstrate an important function for the tumour suppressor RASSF1A in controlling the MDM2–p53 pathway. RASSF1A regulated G1–S cell-cycle progression in a p53-dependent manner and had a function in regulating the MDM2–p53 pathway by promoting MDM2 self-ubiquitination and preventing p53 degradation at an early stage in response to DNA damage. Importantly, RASSF1A is associated with both MDM2 and DAXX in the nucleus, thereby disrupting MDM2–DAXX–HAUSP interactions and enhancing the intrinsic ubiquitin ligase activity of MDM2 (Figure 8D).
When cells are under certain types of stress, MDM2 is prevented from inhibiting p53 function, thus ensuring that p53 can execute an appropriate stress response (Vogelstein et al, 2000). Here, we show that the tumour suppressor RASSF1A is associated with MDM2 and promoted MDM2 destabilization. There is considerable evidence that MDM2 trans- and self-ubiquitinating activities can be differentially regulated through MDM2 interactions with other proteins. For example, MDM2 self-ubiquitination is regulated by MDM2-selective deubiquitination by the ubiquitin hydrolase, HAUSP (Li et al, 2004; Meulmeester et al, 2005). Intriguingly, the balance between self- and p53-ubiquitinating activities of MDM2 can be regulated by the combined actions of DAXX and HAUSP (Tang et al, 2006). Both DAXX and HAUSP stabilize MDM2 and increase MDM2 ubiquitin ligase activity towards p53, and thereby trigger p53 destabilization. Here, we demonstrate that RASSF1A bound to MDM2 and DAXX and disrupted this complex (Figure 6A), resulting in increased MDM2 self-ubiquitination and degradation, and p53 stabilization. By contrast, both siRNA-mediated depletion of RASSF1A and genetic ablation of Rassf1a prevented dissociation of MDM2 from the complex, even after adriamycin treatment (Figure 7C and D). Thus, it is likely that RASSF1A contributes to the dissociation of MDM2 from DAXX and HAUSP, and increases MDM2 self-ubiquitination, at an early time point after DNA damage.
How does RASSF1A prevent DAXX–HAUSP-mediated MDM2 stabilization and stabilize p53 after DNA damage? First, consistent with a previous report (Tang et al, 2006), our study showed that DAXX is a key component of the MDM2–DAXX–HAUSP complex. RASSF1A binds to both the MDM2 C-terminal region, which contains the RING finger domain, and the DAXX N-terminal region, which contains the MDM2- and HAUSP-interacting domain (Figures 4 and 5) (Tang et al, 2006). Thus, RASSF1A may competitively bind to the DAXX–MDM2–HAUSP complex and sequester DAXX from MDM2 and vice versa. Second, the regulation of DAXX–MDM2 dissociation by RASSF1A in response to DNA damage may be more complex than suggested by this simple competition model; other factors may be involved. It has been proposed that MDM2 dissociation from DAXX and HAUSP is partially dependent on ATM kinase (Tang et al, 2006). In addition, transient destabilization of MDM2 appears to depend on the activities of kinases such as ATM, ATR, or DNA-PK, which are activated upon DNA damage (Khosravi et al, 1999; Maya et al, 2001; Stommel and Wahl, 2004). However, because the phosphorylation of MDM2 at Ser395 by ATM kinase is not likely to be essential for MDM2 release from DAXX in response to DNA damage (Tang et al, 2006), it is tempting to speculate that RASSF1A may function as a cofactor for DNA-damage-induced kinases in the regulation of the MDM2–DAXX–HAUSP complex. Intriguingly, we found that the interaction of MDM2 with RASSF1A was slightly increased upon DNA damage, whereas DNA damage led to a decrease in DAXX–MDM2 interactions (Figure 7A). Although the significance is not clear in this context, it is noteworthy that RASSF1A contains a putative phosphorylation site for ATM kinase (Kim et al, 1999). In any case, the functional relationship between RASSF1A and the MDM2–DAXX–HAUSP complex and potential modulation of these interactions, by DNA-damage-induced kinases warrant further investigation. Recently, RASSF1A has been shown to disrupt the Raf1–MST2 kinase complex, thereby enhancing MST2–LATS1 interactions (Matallanas et al, 2007). RASSF1A-mediated LATS1 activation promotes p73-directed transcription of the proapoptotic gene puma. Because the functions of p53 overlap with those of the p53-related proteins, p63 and p73, it is tempting to speculate that RASSF1A-activated MST2–LATS1 kinase also regulates the p53–MDM2 pathway.
RASSF1C has been shown to associate with DAXX in the nucleus (Kitagawa et al, 2006). Upon DNA damage, RASSF1C dissociates from DAXX, translocates to cytoplasmic microtubules, and participates in the activation of SAPK/JNK. However, because RASSF1A remains localized to the nucleus (MS Song and D-S Lim, unpublished data), whereas RASSF1C translocates to the cytoplasm upon DNA damage, RASSF1A and RASSF1C might have distinct functions following genotoxic stress. RASSF1C associated with DAXX in vivo, but interestingly failed to disrupt the MDM2–DAXX complex in vitro and in vivo (Figure 6D and Supplementary Figure S7A). Moreover, RASSF1C did not affect cell-cycle distribution or the stability of MDM2 or p53 (Supplementary Figures S1 and S2). It is not entirely clear why RASSF1C fails to dissociate the MDM2–DAXX complex even it is able to associate with DAXX. However, differences in the N-terminal regions of RASSF1C and RASSF1A led us to speculate that the ability of RASSF1A to interfere with MDM2–DAXX interactions might require both MDM2 binding to the N terminus of RASSF1A and DAXX binding to the C terminus of RASSF1A. Moreover, the interaction of the deubiquitination enzyme, HAUSP, with either DAXX or MDM2 was not affected by exogenous RASSF1C (Supplementary Figure S7B and C), suggesting that RASSF1C alone is insufficient to both completely disrupt the MDM2–DAXX–HAUSP complex and regulate MDM2 and p53 stability.
The tumour suppressor ARF binds to MDM2 and preferentially inhibits MDM2 p53-ubiquitinating activity (Zhang et al, 1998; Sherr and Weber, 2000). The ARF-binding region of MDM2 is distinct from the RASSF1A-binding region, but the E1A-regulated transcription factor, E4F1, physically interacts with ARF and RASSF1A (Rizos et al, 2003; Fenton et al, 2004). Thus, it will be of interest to investigate the effects of RASSF1A on the binding of ARF to MDM2. Because it has been proposed that E4F1 is an atypical p53 ubiquitin ligase that regulates p53 effector functions independently of proteolysis (Le Cam et al, 2006), the effect of RASSF1A on E4F1-mediated p53 activation also warrants further investigation. The RING finger protein, MDMX, inhibits p53 transcriptional activity by directly interacting with p53 (Shvarts et al, 1996; Laurie et al, 2006). MDMX also binds to MDM2, but whether the main effect of this binding is to stabilize MDM2, and thus indirectly inhibit p53, or whether the interaction is more important for MDMX activity by MDM2, remains to be determined. Notably, RASSF1A binds directly to the RING domain of MDM2 but not to that of MDMX (MS Song and D-S Lim, unpublished data). Future studies will be required to address the possible role of RASSF1A in regulating MDMX–MDM2 interactions.
In conclusion, our study demonstrates an important function for RASSF1A in the negative regulation of MDM2. Because MDM2 destabilization is required for a proper p53 response at early time points after DNA damage, the tumour suppressor, RASSF1A, may have an important function in the early stages of the cellular response to genotoxic stress.
Materials and methods
Cell culture
Cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) fetal bovine serum. HCT116 (p53+/+ or p53−/−) cells were kindly provided by B Vogelstein; Mdm2+/+p53−/− and Mdm2−/−p53−/− MEFs were the kind gifts of G Lozano; Rassf1a wild-type and null MEFs were kindly provided by G Pfeifer.
Plasmids and recombinant proteins
RASSF1A, HA–RASSF1A, Flag–RASSF1A, HA–p53, Flag–MDM2, HA–MDM2, HA–DAXX, GST–RASSF1A, GST–RASSF1C, GST–MDM2 and GST–MDM2(ΔRING) were constructed by subcloning the corresponding cDNAs into untagged pcDNA3.1 or pcDNA3.1 (Invitrogen) containing the DNA sequence for an N-terminal HA or Flag tag, or into the pGEX vector. The plasmid pGEX-ubiquitin was kindly provided by AM Weissman; His6-ubiquitin was a gift from D Lim; Flag–DAXX was kindly provided by E Choi; Myc–HAUSP was a gift from K Pak.
Antibodies
Guinea-pig polyclonal antibodies to RASSF1A were raised against a GST fusion protein containing an N-terminal fragment of human RASSF1A (residues 1–119). Specific antibodies were affinity purified using the RASSF1A antigen. Also used antibodies were against: HA (Roche); Flag and β-actin (Sigma); ubiquitin and MDM2 (SMP14) (BD Pharmingen); cyclin A, p53 (DO-1), p21, MDM2 (SMP14), GST, HDAC1, β-tubulin, and DAXX (Santa Cruz Biotechnology); Myc (Cell Signaling); p53 (Ab-6) and p53 (Ab-1) (Calbiochem); HAUSP and MDMX (Bethyl).
RNAi
The RASSF1A siRNA sequence was 5′-GACCUCUGUGGCGACUUCA-3′. The DNA sequence for this siRNA was inserted into the plasmid pSUPER (Oligoengine) and introduced into cells by transfection using the Effectene reagent (Qiagen).
Immunoblot analysis, immunoprecipitation, in vitro binding assays, and immunofluorescence
Immunoblot and immunoprecipitation analyses were performed as described previously (Song et al, 2004). For in vitro binding assays, in vitro-translated 35S-labelled products were generated using the TNT-Coupled System (Promega). The products were incubated with anti-Flag or anti-HA antibodies in binding buffer (50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 0.5% (w/v) Triton X-100, phosphatase inhibitors, and protease inhibitors) for 2 h at 4°C. The beads were washed four times with binding buffer, and bound 35S-labelled products were detected by SDS–PAGE and autoradiography. For immunofluorescence, U2OS cells were permeabilized with 0.2% (w/v) Triton X-100 for 10 min, fixed with paraformaldehyde, and blocked with 10% (v/v) normal goat serum, followed by consecutive exposure to primary and secondary antibodies with intervening washes. Slides were mounted with a medium containing 4,6-diamidino-2-phenylindole (DAPI) and imaged using a Carl Zeiss LSM510 META microscope (C-Apochromat × 40/1.2W) equipped with a charge-coupled device and a PMT detector at the side port. The green and red channels used 488 and 543 nm argon laser lines for excitation. Data were processed with LSM 510 META System software.
Ubiquitination assays
To examine the effects of RASSF1A on ubiquitylation of p53 or MDM2 in vivo, cells were lysed by incubation for 10 min at 95°C with two volumes of TBS (10 mM Tris–HCl (pH 7.5), 150 mM NaCl) containing 2% (w/v) SDS. After adding eight volumes of 1% (w/v) Triton X-100 in TBS, the lysates were sonicated for 2 min and then incubated with protein G-agarose beads. The beads were removed by centrifugation, and the lysates were immunoprecipitated with protein G-agarose-coupled anti-p53 (DO-1) or anti-MDM2 (SMP14) antibodies. The beads were first washed with 0.5 M LiCl in TBS and then twice with TBS, and boiled. Proteins were separated by SDS–PAGE and analysed by immunoblotting using an anti-ubiquitin antibody. Alternatively, cells were transfected with a vector for His6-ubiquitin and His6-proteins were purified from cell lysates using a Ni2+-NTA spin column (Qiagen) under denaturing conditions. For analysis of MDM2 self-ubiquitination in vitro, purified GST–MDM2 or GST–MDM2(ΔRING) (250 ng) was incubated for 90 min at 30°C with E1, Ubc5b (Boston Biochem), an ATP-regenerating system (7.5 mM creatine phosphate, 1 mM ATP, 1 mM MgCl2, 0.1 mM EGTA, rabbit creatine phosphokinase type I (30 U/ml; Sigma)), and 32P-labelled ubiquitin (2 × 104 c.p.m.) prepared with GST ubiquitin, as described previously (Fang et al, 2000). The extent of ubiquitination was then analysed by SDS–PAGE and autoradiography.
Cell-cycle analysis
Analysis of DNA content during cell-cycle progression was performed as described previously (Song et al, 2004). For analysis of BrdU incorporation, cells were labelled with 10 μM BrdU (Sigma) for 1 h, fixed with 70% (v/v) ethanol, incubated with 2 M HCl for 20 min, washed with 0.1 M sodium borate (pH 8.5) and then with PBS containing 0.5% (w/v) BSA. Incorporation of BrdU was detected by immunostaining or flow cytometry using an anti-BrdU antibody (BD Pharmingen) and the percentage of BrdU-labelled nuclei was quantified.
Supplementary Material
Supplementary Figure S1–S9
Acknowledgments
This study was supported by a grant from the Korea Research Foundation (KRF-2005-C00093), a Nuclear Research Grant, the 21st Century Frontier Functional Human Genome Project, and the Korea National Cancer Center Program.
References
- Agathanggelou A, Cooper WN, Latif F (2005) Role of the Ras-association domain family 1 tumor suppressor gene in human cancers. Cancer Res 65: 3497–3508 [DOI] [PubMed] [Google Scholar]
- Baksh S, Tommasi S, Fenton S, Yu VC, Martins LM, Pfeifer GP, Latif F, Downward J, Neel BG (2005) The tumor suppressor RASSF1A and MAP-1 link death receptor signaling to Bax conformational change and cell death. Mol Cell 18: 637–650 [DOI] [PubMed] [Google Scholar]
- Brooks CL, Gu W (2006) p53 ubiquitination: Mdm2 and beyond. Mol Cell 21: 307–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B (1998) Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282: 1497–1501 [DOI] [PubMed] [Google Scholar]
- Cummins JM, Rago C, Kohli M, Kinzler KW, Lengauer C, Vogelstein B (2004) Tumour suppression: disruption of HAUSP gene stabilizes p53. Nature 428: 1 p following 486 [DOI] [PubMed] [Google Scholar]
- Dai MS, Lu H (2004) Inhibition of MDM2-mediated p53 ubiquitination and degradation by ribosomal protein L5. J Biol Chem 279: 44475–44482 [DOI] [PubMed] [Google Scholar]
- Dai MS, Zeng SX, Jin Y, Sun XX, David L, Lu H (2004) Ribosomal protein L23 activates p53 by inhibiting MDM2 function in response to ribosomal perturbation but not to translation inhibition. Mol Cell Biol 24: 7654–7668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammann R, Li C, Yoon JH, Chin PL, Bates S, Pfeifer GP (2000) Epigenetic inactivation of a RAS association domain family protein from the lung tumour suppressor locus 3p21.3. Nat Genet 25: 315–319 [DOI] [PubMed] [Google Scholar]
- Donninger H, Vos MD, Clark GJ (2007) The RASSF1A tumor suppressor. J Cell Sci 120: 3163–3172 [DOI] [PubMed] [Google Scholar]
- Fang S, Jensen JP, Ludwig RL, Vousden KH, Weissman AM (2000) Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. J Biol Chem 275: 8945–8951 [DOI] [PubMed] [Google Scholar]
- Fenton SL, Dallol A, Agathanggelou A, Hesson L, Ahmed-Choudhury J, Baksh S, Sardet C, Dammann R, Minna JD, Downward J, Maher ER, Latif F (2004) Identification of the E1A-regulated transcription factor p120 E4F as an interacting partner of the RASSF1A candidate tumor suppressor gene. Cancer Res 64: 102–107 [DOI] [PubMed] [Google Scholar]
- Guo C, Tommasi S, Liu L, Yee JK, Dammann R, Pfeifer GP (2007) RASSF1A is part of a complex similar to the Drosophila Hippo/Salvador/Lats tumor-suppressor network. Curr Biol 17: 700–705 [DOI] [PubMed] [Google Scholar]
- Haupt Y, Maya R, Kazaz A, Oren M (1997) Mdm2 promotes the rapid degradation of p53. Nature 387: 296–299 [DOI] [PubMed] [Google Scholar]
- Higashitsuji H, Itoh K, Sakurai T, Nagao T, Sumitomo Y, Masuda T, Dawson S, Shimada Y, Mayer RJ, Fujita J (2005) The oncoprotein gankyrin binds to MDM2/HDM2, enhancing ubiquitylation and degradation of p53. Cancer Cell 8: 75–87 [DOI] [PubMed] [Google Scholar]
- Jin A, Itahana K, O'Keefe K, Zhang Y (2004) Inhibition of HDM2 and activation of p53 by ribosomal protein L23. Mol Cell Biol 24: 7669–7680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo T, Weber JD, Zambetti G, Zindy F, Roussel MF, Sherr CJ (1998) Functional and physical interactions of the ARF tumor suppressor with p53 and Mdm2. Proc Natl Acad Sci USA 95: 8292–8297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi R, Maya R, Gottlieb T, Oren M, Shiloh Y, Shkedy D (1999) Rapid ATM-dependent phosphorylation of MDM2 precedes p53 accumulation in response to DNA damage. Proc Natl Acad Sci USA 96: 14973–14977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ST, Lim DS, Canman CE, Kastan MB (1999) Substrate specificities and identification of putative substrates of ATM kinase family members. J Biol Chem 274: 37538–37543 [DOI] [PubMed] [Google Scholar]
- Kitagawa D, Kajiho H, Negishi T, Ura S, Watanabe T, Wada T, Ichijo H, Katada T, Nishina H (2006) Release of RASSF1C from the nucleus by Daxx degradation links DNA damage and SAPK/JNK activation. EMBO J 25: 3286–3297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubbutat MH, Jones SN, Vousden KH (1997) Regulation of p53 stability by Mdm2. Nature 387: 299–303 [DOI] [PubMed] [Google Scholar]
- Laurie NA, Donovan SL, Shih CS, Zhang J, Mills N, Fuller C, Teunisse A, Lam S, Ramos Y, Mohan A, Johnson D, Wilson M, Rodriguez-Galindo C, Quarto M, Francoz S, Mendrysa SM, Guy RK, Marine JC, Jochemsen AG, Dyer MA (2006) Inactivation of the p53 pathway in retinoblastoma. Nature 444: 61–66 [DOI] [PubMed] [Google Scholar]
- Le Cam L, Linares LK, Paul C, Julien E, Lacroix M, Hatchi E, Triboulet R, Bossis G, Shmueli A, Rodriguez MS, Coux O, Sardet C (2006) E4F1 is an atypical ubiquitin ligase that modulates p53 effector functions independently of degradation. Cell 127: 775–788 [DOI] [PubMed] [Google Scholar]
- Li M, Brooks CL, Kon N, Gu W (2004) A dynamic role of HAUSP in the p53–Mdm2 pathway. Mol Cell 13: 879–886 [DOI] [PubMed] [Google Scholar]
- Li M, Chen D, Shiloh A, Luo J, Nikolaev AY, Qin J, Gu W (2002) Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature 416: 648–653 [DOI] [PubMed] [Google Scholar]
- Linares LK, Hengstermann A, Ciechanover A, Muller S, Scheffner M (2003) HdmX stimulates Hdm2-mediated ubiquitination and degradation of p53. Proc Natl Acad Sci USA 100: 12009–12014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Tommasi S, Lee DH, Dammann R, Pfeifer GP (2003) Control of microtubule stability by the RASSF1A tumor suppressor. Oncogene 22: 8125–8136 [DOI] [PubMed] [Google Scholar]
- Lohrum MA, Ludwig RL, Kubbutat MH, Hanlon M, Vousden KH (2003) Regulation of HDM2 activity by the ribosomal protein L11. Cancer Cell 3: 577–587 [DOI] [PubMed] [Google Scholar]
- Marine JC, Jochemsen AG (2004) Mdmx and Mdm2: brothers in arms? Cell Cycle 3: 900–904 [PubMed] [Google Scholar]
- Matallanas D, Romano D, Yee K, Meissl K, Kucerova L, Piazzolla D, Baccarini M, Vass JK, Kolch W, O'neill E (2007) RASSF1A elicits apoptosis through an MST2 pathway directing proapoptotic transcription by the p73 tumor suppressor protein. Mol Cell 27: 962–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maya R, Balass M, Kim ST, Shkedy D, Leal JF, Shifman O, Moas M, Buschmann T, Ronai Z, Shiloh Y, Kastan MB, Katzir E, Oren M (2001) ATM-dependent phosphorylation of Mdm2 on serine 395: role in p53 activation by DNA damage. Genes Dev 15: 1067–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulmeester E, Maurice MM, Boutell C, Teunisse AF, Ovaa H, Abraham TE, Dirks RW, Jochemsen AG (2005) Loss of HAUSP-mediated deubiquitination contributes to DNA damage-induced destabilization of Hdmx and Hdm2. Mol Cell 18: 565–576 [DOI] [PubMed] [Google Scholar]
- Michael D, Oren M (2003) The p53–Mdm2 module and the ubiquitin system. Semin Cancer Biol 13: 49–58 [DOI] [PubMed] [Google Scholar]
- Michaelson JS, Bader D, Kuo F, Kozak C, Leder P (1999) Loss of Daxx, a promiscuously interacting protein, results in extensive apoptosis in early mouse development. Genes Dev 13: 1918–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh HJ, Lee KK, Song SJ, Jin MS, Song MS, Lee JH, Im CR, Lee JO, Yonehara S, Lim DS (2006) Role of the tumor suppressor RASSF1A in Mst1-mediated apoptosis. Cancer Res 66: 2562–2569 [DOI] [PubMed] [Google Scholar]
- Parant J, Chavez-Reyes A, Little NA, Yan W, Reinke V, Jochemsen AG, Lozano G (2001) Rescue of embryonic lethality in Mdm4-null mice by loss of Trp53 suggests a nonoverlapping pathway with MDM2 to regulate p53. Nat Genet 29: 92–95 [DOI] [PubMed] [Google Scholar]
- Pomerantz J, Schreiber-Agus N, Liégeois NJ, Silverman A, Alland L, Chin L, Potes J, Chen K, Orlow I, Lee HW, Cordon-Cardo C, DePinho RA (1998) The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2's inhibition of p53. Cell 92: 713–723 [DOI] [PubMed] [Google Scholar]
- Prives C (1998) Signaling to p53: breaking the MDM2–p53 circuit. Cell 95: 5–8 [DOI] [PubMed] [Google Scholar]
- Rizos H, Diefenbach E, Badhwar P, Woodruff S, Becker TM, Rooney RJ, Kefford RF (2003) Association of p14ARF with the p120E4F transcriptional repressor enhances cell cycle inhibition. J Biol Chem 278: 4981–4989 [DOI] [PubMed] [Google Scholar]
- Rong R, Jin W, Zhang J, Sheikh MS, Huang Y (2004) Tumor suppressor RASSF1A is a microtubule-binding protein that stabilizes microtubules and induces G2/M arrest. Oncogene 23: 8216–8230 [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Weber JD (2000) The ARF/p53 pathway. Curr Opin Genet Dev 10: 94–99 [DOI] [PubMed] [Google Scholar]
- Shivakumar L, Minna J, Sakamaki T, Pestell R, White MA (2002) The RASSF1A tumor suppressor blocks cell cycle progression and inhibits cyclin D1 accumulation. Mol Cell Biol 22: 4309–4318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvarts A, Steegenga WT, Riteco N, van Laar T, Dekker P, Bazuine M, van Ham RC, van der Houven van Oordt W, Hateboer G, van der Eb AJ, Jochemsen AG (1996) MDMX: a novel p53-binding protein with some functional properties of MDM2. EMBO J 15: 5349–5357 [PMC free article] [PubMed] [Google Scholar]
- Song MS, Song SJ, Ayad NG, Chang JS, Lee JH, Hong HK, Lee H, Choi N, Kim J, Kim H, Kim JW, Choi EJ, Kirschner MW, Lim DS (2004) The tumour suppressor RASSF1A regulates mitosis by inhibiting the APC–Cdc20 complex. Nat Cell Biol 6: 129–137 [DOI] [PubMed] [Google Scholar]
- Song MS, Song SJ, Kim SJ, Nakayama K, Nakayama KI, Lim DS (2008) Skp2 regulates the antiproliferative function of the tumor suppressor RASSF1A via ubiquitin-mediated degradation at the G(1)–S transition. Oncogene 17: 3176–3185 [DOI] [PubMed] [Google Scholar]
- Stommel JM, Wahl GM (2004) Accelerated MDM2 auto-degradation induced by DNA-damage kinases is required for p53 activation. EMBO J 23: 1547–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui G, Affar el B, Shi Y, Brignone C, Wall NR, Yin P, Donohoe M, Luke MP, Calvo D, Grossman SR (2004) Yin Yang 1 is a negative regulator of p53. Cell 117: 859–872 [DOI] [PubMed] [Google Scholar]
- Tang J, Qu LK, Zhang J, Wang W, Michaelson JS, Degenhardt YY, El-Deiry WS, Yang X (2006) Critical role for Daxx in regulating Mdm2. Nat Cell Biol 8: 855–862 [DOI] [PubMed] [Google Scholar]
- Toledo F, Wahl GM (2006) Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer 6: 909–923 [DOI] [PubMed] [Google Scholar]
- Tommasi S, Dammann R, Zhang Z, Wang Y, Liu L, Tsark WM, Wilczynski SP, Li J, You M, Pfeifer GP (2005) Tumor susceptibility of Rassf1a knockout mice. Cancer Res 65: 92–98 [PubMed] [Google Scholar]
- van der Weyden L, Tachibana KK, Gonzalez MA, Adams DJ, Ng BL, Petty R, Venkitaraman AR, Arends MJ, Bradley A (2005) The RASSF1A isoform of RASSF1 promotes microtubule stability and suppresses tumorigenesis. Mol Cell Biol 25: 8356–8367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B, Lane D, Levine AJ (2000) Surfing the p53 network. Nature 408: 307–310 [DOI] [PubMed] [Google Scholar]
- Vos MD, Martinez A, Elam C, Dallol A, Taylor BJ, Latif F, Clark GJ (2004) A role for the RASSF1A tumor suppressor in the regulation of tubulin polymerization and genomic stability. Cancer Res 64: 4244–4250 [DOI] [PubMed] [Google Scholar]
- Yang X, Khosravi-Far R, Chang HY, Baltimore D (1997) Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell 89: 1067–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wolf GW, Bhat K, Jin A, Allio T, Burkhart WA, Xiong Y (2003) Ribosomal protein L11 negatively regulates oncoprotein MDM2 and mediates a p53-dependent ribosomal-stress checkpoint pathway. Mol Cell Biol 23: 8902–8912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xiong Y, Yarbrough WG (1998) ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell 92: 725–734 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1–S9