Abstract
Since the amino acid derivative N-acetylaspartate (NAA) is almost exclusive to neuronal cells in the adult mammalian brain and its concentration has shown local (or global) abnormalities in most focal (or diffuse) neurological diseases, it is considered a specific neuronal marker. Yet despite its biological and clinical prominence, the relative NAA concentration in the gray and white matter (GM, WM) remains controversial, with each reported to be higher than, equal to, or less than the other. To help resolve the controversy and importantly, access the NAA in both compartments in their entirety, we introduce a new approach to distinguish and quantify the whole-brain average GM and WM NAA concentration by integrating MR-image segmentation, localized and non-localized quantitative 1H-MRS. We demonstrate and validate the method in ten healthy volunteers (5 women) 27±6 years old (mean ± standard-deviation) at 1.5 T. The results show that the healthy adult human brain comprises significantly less WM, 39±3%, than GM 60±4% by volume (p<0.01). Furthermore, the average NAA concentration, in the WM, 9.5±1.0 mM, is significantly lower than in GM, 14.3±1.1 mM (p<0.01) with no gender differences (p>0.5).
Keywords: Absolute quantification, Brain, gray matter, N-acetylaspartate (NAA), proton magnetic resonance spectroscopy (1H-MRS), white matter
Introduction
The amino acid derivative N-acetylaspartate (NAA, see Fig. 1) was first described over fifty years ago (Tallan et al., 1956). At up to 0.1% of the brain tissue wet weight, NAA is the second most abundant amino acid in the human central nervous system (CNS) after glutamate (Baslow, 2003). The singlet resonance of the protons of its N-acetyl group is the most prominent peak in the proton MR spectrum (1H-MRS) of the brain, as shown in Fig. 1, making it straightforward to quantify non-invasively in vivo (Jansen et al., 2006). Since in the mature adult brain NAA is almost exclusively localized in neurons and their processes (Baslow, 2003; Simmons et al., 1991) and although its exact role(s) there are still unknown (Moffett et al., 2007), it is widely considered a putative marker of their density, integrity and viability (Arnold et al., 2001).
Fig. 1.
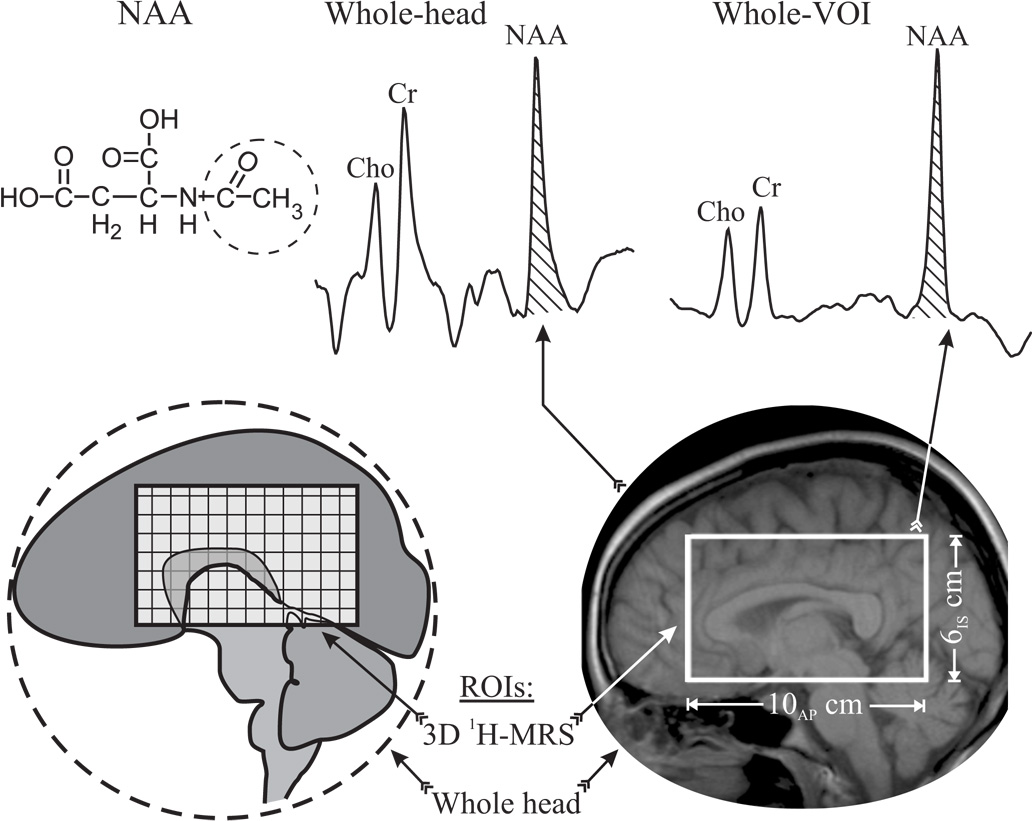
Top, left: Schematic structure of NAA with the N-acetyl group giving rise to the singlet at 2.01 ppm circled (dashed line). Center: Whole-head 1H-spectrum. The NAA peak used for integration in Eq. [6] is hatched. Right: Sum of 640 aligned spectra from all 640 voxels inside the VOI with NAA area used for quantification in Eq. [7], hatched. Note the excellent SNR and spectral resolution of the sum due to its pre-alignment. Bottom: Schematic depiction of the two volumes used: Whole brain (circled region) and the 10AP×8LR×6IS cm3 PRESS VOI used to set up Eq. [2] and Eq. [3], respectively.
Indeed, among the resonances identified in neuro-1H-MRS, none has yielded more diagnostic information than the NAA. Its regional (or global) concentration decline has been shown in most focal (or diffuse) CNS disorders (Ross and Bluml, 2001; Schuff et al., 2006). To better identify and quantify diffuse abnormalities, however, the global NAA concentration must be known in the various compartments of the healthy CNS (Jansen et al., 2006), which to a first approximation comprises gray and white matter and cerebrospinal fluid (GM, WM, CSF) (Clark et al., 2006; Pfefferbaum et al., 1999b).
Despite its incontrovertible prominence, however, the NAA concentration of either compartment of the healthy brain remains controversial. For example, a partial sample of the literature shows NAA levels to be 1.2 – 1.5 times higher in GM by some (Doyle et al., 1995; Lim and Spielman, 1997; McLean and Barker, 2006; Narayana et al., 1989; Noworolski et al., 1999; Wang and Li, 1998). In contrast, others report the NAA concentration to be more than 1.1 fold lower in GM than in WM (Gideon et al., 1995; Hetherington et al., 1996; Schuff et al., 2001; Soher et al., 1996; Tedeschi et al., 1995), while others find it to not differ significantly between the two compartments (Kreis et al., 1993; Lopez-Villegas et al., 1996; Michaelis et al., 1993).
The discrepancy may be due (at least in part) to a confluence of three problem areas: First, neither single-voxel nor two or even three-dimensional (3D) 1H-MRS presently cover the entire brain. Second, the sensitivity of 1H-MRS is insufficient to fully resolve GM from WM. Finally, most cortical GM is difficult to access since it is only 1 – 4 mm thick, tortuous and adjacent to bone marrow and subcutaneous lipids whose signals may obscure the NAA’s (Moonen et al., 1992). Consequently, the volume-of-interest (VOI) must often be away from the skull and even careful placement cannot eliminate partial GM/WM volume effects. Although some 1H-MRS studies applied sophisticated tissue segmentation to account for it, they neither eliminated it nor covered the whole brain (Stadlbauer et al., 2004; Weber-Fahr et al., 2002). These issues combine to make the assessment of the total loads of the neuronal and axonal dysfunctions (in the GM and WM) of diffuse neurological disorders inaccessible to the current 1H-MRS methodologies.
To address the above issues, the goal of this paper is to introduce a new method that integrates MRI segmentation, non-localizing whole brain NAA (WBNAA) and 3D 1H-MRS quantification. to distinguish and quantify the average global NAA concentrations in the entire GM and WM moieties of healthy volunteers. The rationale for such cohort is that absent a gold standard then for purposes of validation, the precision must be established. Specifically, a reliable method should yield the same WM and GM NAA concentration across all healthy individuals.
Theory
As indicated above, lipid signal contamination renders 1H-MRS unreliable near the skull (Moonen et al., 1992). Although the WBNAA method was developed specifically to overcome this problem, it does so at the expense of localization (Gonen et al., 1998b), yielding the total amount of NAA in the brain,
| [1] |
CB is the average NAA concentration over the entire brain tissue volume, VB. QNAA is obtained with WBNAA to within ±6% (Benedetti et al., 2007; Gonen et al., 1998b) and VB, from MRI-segmentation, to within ±0.5% (Clark et al., 2006; Mikheev et al., 2008). Unfortunately, the WM contributions to QNAA, cannot be separated from the GM’s since,
| [2] |
where VWM and VGM are the WM and GM volumes (VB=VWM+VGM) and FWM =VWM/VB the WM fraction. Therefore, the two unknowns, CWM and CGM, cannot both be derived from Eq. [2].
This problem can be resolved if a second equation with the same unknowns can be obtained from another VOI, vVOI, in the same brain, as shown in Fig. 1. Then, in analogy with Eq. [2],
| [3] |
where qNAA is the amount of NAA in vVOI, vWM, vGM its WM and GM volumes and fWM = vWM / vVOI, its WM fraction. Eq. [2] and Eq. [3] are linearly independent if in their vector form,
| [4] |
the determinant of the 2×2 matrix ≠0. Fortunately, due to the brain’s GM and WM heterogeneity, this condition is easily met if vb is placed judiciously to comprise different WM fractions than VB, i.e., FWM≠ fWM (cf. Fig. 1 and Fig. 2). Then, Eq. [4] has a unique solution for the average global NAA concentrations of each moiety,
| [5] |
Fig. 2.
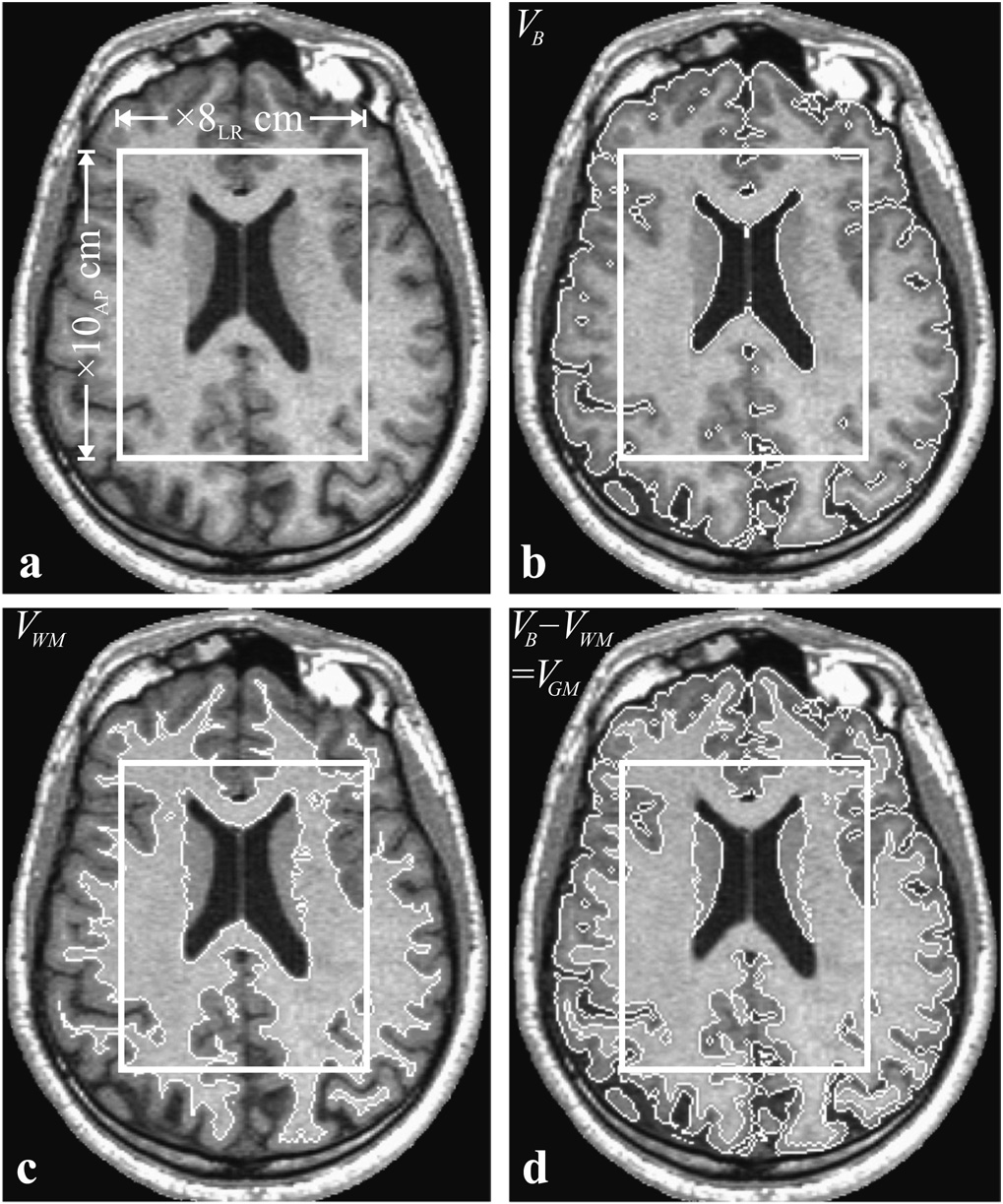
Top, left, a: Axial T1-weighted image showing the geometry and placement of the PRESS 10AP×8LR cm2 VOI (solid frame). Top, right, b: Same as a but with the tissue mask generated by the segmentation software (white trace). Note the accurate tissue/CSF differentiation. Bottom, left, c: Same slice with only WM segmented and highlighted (all pixels intensities above 0.55IWM). Bottom, right, d: GM mask, obtained as the b – c difference highlighted. Note the faithful tracing of the cortical ribbon and caudate at this level. All volumetry: VB, VWM, VGM, vVOI, vWM, and vGM described in the text and compiled in Table 1 were derived by counting the pixels within the corresponding masks.
Materials and Methods
Human subjects
Ten healthy subjects (5 women and 5 men) mean age 28.2±5.5 (range 22 – 38) years old, with no history of neurological disorders prior to the scan and unremarkable MRI afterwards, were recruited. Their demographics are given in Table 1. All were briefed on the imaging procedure they were to undergo and gave Institutional Review Board approved written informed consent.
Table 1.
Age and gender of the cohort and the various volumetric, “V”, “v” (global and VOI) and quantitative “Q” and “q” NAA metrics. The average global NAA concentration in the GM (CGM) and WM (CGM) compartments of each individual are estimated from Eq. [5] in the two rightmost columns (bold). Note that the average global CGM is significantly higher than CWM (p<0.001).
| Subject | aAge/sex | bVB | bVWM | bVGM | bvVOI | bvWM | bvGM | cQNAA | cqNAA | dCWM | dCGM |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 22/M | 1466 | 557 | 906 | 457 | 319 | 124 | 17.8 | 4.8 | 8.9 | 14.1 |
| 2 | 22/F | 1238 | 461 | 770 | 451 | 310 | 141 | 15.00 | 4.8 | 9.0 | 14.0 |
| 3 | 24/F | 1098 | 650 | 410 | 458 | 309 | 149 | 13.08 | 4.9 | 9.6 | 13.3 |
| 4 | 26/M | 1289 | 434 | 848 | 453 | 322 | 130 | 16.2 | 4.7 | 8.6 | 14.6 |
| 5 | 27/M | 1233 | 521 | 712 | 447 | 295 | 146 | 13.5 | 5.0 | 9.8 | 13.7 |
| 6 | 27/M | 1439 | 650 | 789 | 452 | 340 | 128 | 16.8 | 4.7 | 9.4 | 13.8 |
| 7 | 28/F | 1331 | 596 | 735 | 447 | 319 | 126 | 15.2 | 5.3 | 11.2 | 13.4 |
| 8 | 32/F | 1212 | 487 | 725 | 440 | 322 | 118 | 15.3 | 5.1 | 10.8 | 13.5 |
| 9 | 36/M | 1492 | 532 | 955 | 453 | 336 | 116 | 19.6 | 4.6 | 8.2 | 15.9 |
| 10 | 38/F | 1198 | 473 | 725 | 452 | 315 | 141 | 16.5 | 5.3 | 9.8 | 16.4 |
| Avg±SD | 28.2±5.5 | 1300±130 | 536±76 | 758±148 | 451±5 | 319±13 | 132±12 | 15.9±2.0 | 4.9±0.2 | 9.5±1.0 | 14.3±1.1 |
Units: years
Units: cm3
Units: millimoles
Units: millimolar (mM).
MRI – segmentation and volumetry
All measurements were done in a 1.5 T whole-body imager (Siemens AG, Erlangen, Germany) using its standard circularly polarized head-coil. MRI comprised T1-weighted MP-RAGE: TE/TR/TI: 7.0/14.7/300 ms; 128 slices 1.5 mm thick each, 256×256 matrix, 210×210 mm2 field of view. Image segmentation, using the MIDAS package (De Santi et al., 2001), started by placing a “seed” region in the periventricular WM, to yield its signal intensity, IWM. Following selection of all the pixels at or above 0.55IWM, a brain mask was constructed for each slice in three steps: (i) morphological erosion, (ii) recursive region growth retaining pixels connected to the “seed” region and (iii) morphological inflation to reverse the effect of erosion. An example of the result is shown in Fig. 2b. Pixels of intensity below 0.55IWM were defined as CSF. The precision of this approach has been described previously (Rusinek and Chandra, 1993) and more recently as applied to T1-weighted imaging at 3.4% (Mikheev et al., 2008).
Next, the brain masks were truncated at the foramen magnum to incorporate the brain stem and cerebellum but not the cord. The WM mask, e.g., Fig. 2c, was constructed as the subset of all pixels with signal intensity over 0.87IWM, a value halfway between mean GM and WM for this sequence. The GM mask was the difference between the all-tissue and WM masks (cf. Fig. 2d). The same procedure was also repeated in the VOI. Finally, VB, VWM, VGM, vVOI, vWM, and vGM of Eq. [1] – Eq. [3] were obtained by multiplying the number of pixels in their masks by their volume.
MRS – Whole brain NAA (WBNAA) quantification
After shimming to a 12±2 Hz whole-head water linewidth, QNAA of Eq. [1] was obtained with non-localizing, non-echo (TE/TI/TR= 0.0/0.97/10.0 s) 1H-MRS (Gonen et al., 1998b). Since the TR»T1 and TE≈0 the sequence is insensitivity to T1 and T2 variations. Quantification was done against a reference 3 L sphere of 1.5×10−2 mole NAA in water. Subject and reference NAA peak areas, SS and SR, were integrated and QNAA obtained as (Soher et al., 1996),
| [6] |
are the radio-frequency voltage into 50 Ω for non-selective 1 ms 180° inversion on the reference and subject, reflecting their coil loading, i.e., relative sensitivity. Note that although the peak at 2.01 ppm also comprises N-acetylaspartylglutamate and other acetyl-bearing species, since over 90% of it arises from the NAA (Baslow, 2003) we refer to it as such for simplicity.
Localized 3D-1H MRS quantification
A PRESS based 3D 1H-MRS (TE/TR =135/1600 ms) was used to excite the image-guided 8 cm left-right (LR) ×10 cm anterior-posterior (AP) ×6 cm inferior-superior (IS) = 480 cm3 VOI centered on the corpus callosum, as shown in Fig. 1 and Fig 2. It was partitioned with 3D hybrid of 1D-8th order Hadamard with 2D 16LR×16AP chemical shift imaging (CSI) into 8LR ×10AP×8IS = 640 voxels a nominal 0.75 cm3 each (Gonen et al., 1998a). The protocol took under 90 minutes.
The 1H-MRS signals were apodized with a 2 Hz Lorentzian, Fourier transformed along LR, AP and time, Hadamard transformed in the IS direction and automatic frequency and phase corrected in each voxel (Gonen et al., 1998a). The relative NAA amount in the jth voxel of each subject, Sj, was estimated from its peak area using parametric spectral modeling and least-squares optimization (Soher et al., 1998). All Sjs were scaled into absolute, qjs, with phantom replacement, as described above (Soher et al., 1996),
| [7] |
where SR is the average phantom voxel NAA peak area. The qjs were adjusted for relaxation time difference between the phantom in vitro and in vivo: T1vitro=T1 vivo=1.4 s, T2 vitro/T2 vivo ≈0.75/0.43 s (Inglese et al., 2003),
| [8] |
Finally, all 640 VOI voxels’ qjs, were summed to yield qNAA of Eq. [3], as shown in Fig. 1. The summation post frequency alignment improves both the SNR and spectral resolution, as shown in Fig. 1, increasing the precision of metabolic quantification (Inglese et al., 2003). Note that this approach assumes that a single set of T1 and T2 values are sufficient for accurate quantification. For the former, it has been shown that for TR≈1.2·TI the signal, Sj in Eq. [6], varies <5% for to up to ±40% variations in T1 (Goelman et al., 2006); and for the latter use of a single T2 (GM and WM average) for quantification leads to Sj variations of less than 10% (Zaaraoui et al., 2007).
Statistical analyses
An exact Wilcoxon matched-pairs signed rank test was used to compare the GM and WM fractions, FWM versus FGM of the total brain volume and their NAA concentrations CWM with CGM. An exact Wilcoxon rank sum test was used to compare these metrics between the genders. Results were declared significant if associated with a two-sided p value of less than 0.01.
Results
Brain volumetry
Global brain, GM and WM volumes, VB, VWM, VGM, as well as their counterparts in the VOI, vWM, vGM, and the corresponding NAA amounts, QNAA and qNAA, are given in Table 1 and their distributions shown in Fig. 3. Note that the global FWM = 39±3% fraction (average ± standard-deviation) is indeed much different than the fWM = 70±4% in the VOI, as required for a unique solution of Eq. [4]. Furthermore, the global WM fraction, 39±3%, in this cohort is statistically different from the GM’s 60±4% with no significant sex differences (p>0.5), as seen in Fig. 3. These data provide 95% confidence that the gender difference in median volume is no greater than 163 cm3 for VWM and 438 cm3 for the VGM.
Fig. 3.
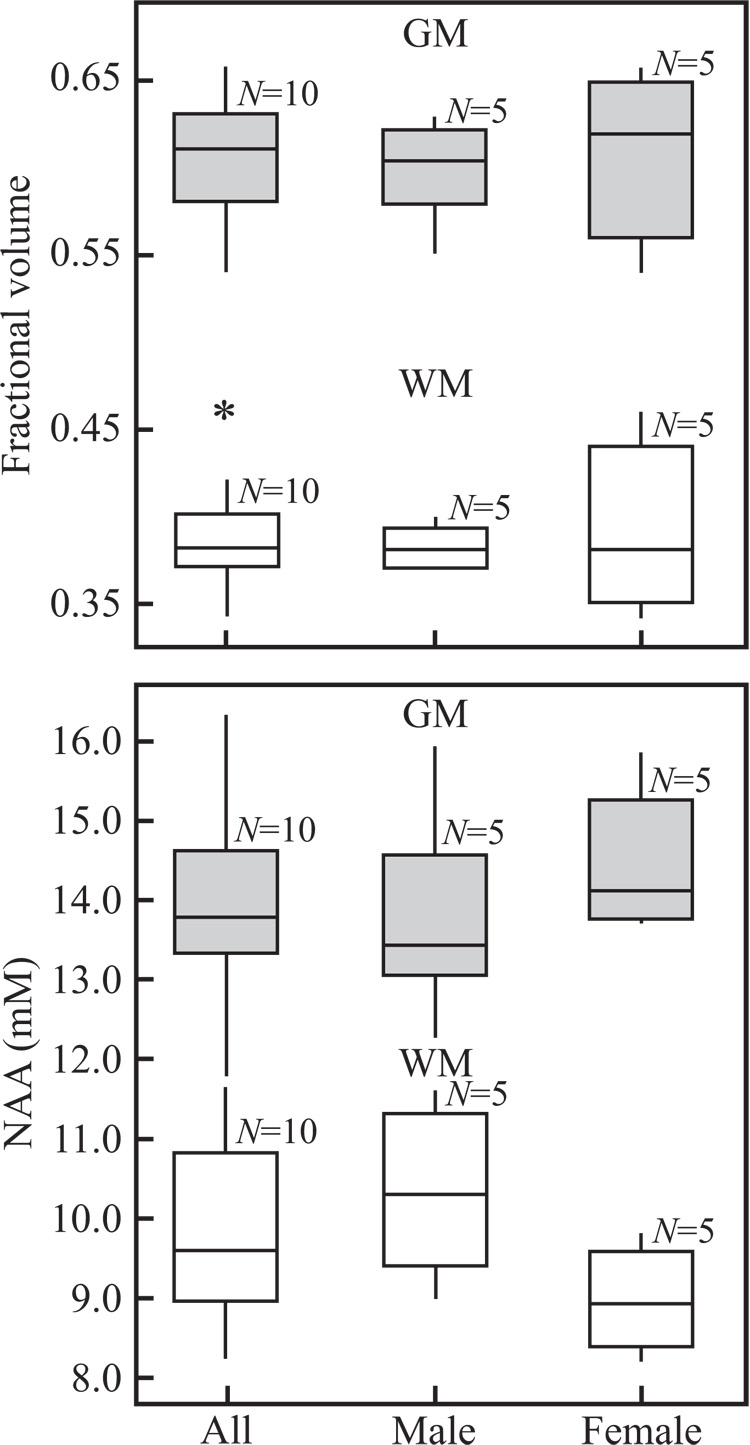
Top: Box plots displaying the 25th and 75th percentiles (box), median (line), 5th and 95th percentiles (whiskers) and outliers (*) of the GM and WM brain volume fraction distributions. Note the significant FWM vs. FGM difference but not between the genders. Bottom: Box plots of the average global NAA concentration in GM and WM, CWM, CGM, in millimoles overall and of males versus females. Note the significant concentrations difference between the compartments, but not between the genders.
Brain NAA concentration
The NAA concentrations, derived from Eq. [5] using the whole brain and VOI NAA amounts and the respective tissue fractions, are given in Table 1 and their distributions shown in Fig. 3. Overall the average global WM NAA concentration, 9.5±1.0 mM, is significantly lower than the GM 14.3±1.1 mM (p<0.001) but with no gender differences (p>0.56), as seen in Fig. 3. These data provide 95% confidence that the gender difference in median NAA concentration is no greater than 2.6 mM for the CWM and 2.4 mM for the GGM.
Discussion
While WBNAA provides an objective specific measure of disease burden over the entire brain, it does so at the cost of complete loss of information on the regional distribution and severity of the pathological processes (Gonen et al., 1998b). Although this is nevertheless useful for monitoring several widespread whole-brain diffuse neurological disorders, e.g., multiple sclerosis, HIV and trauma (Rigotti et al., 2007), other diseases involve predominantly the GM, e.g., dementias, or the WM, e.g., leukodystrophies (Kingsley et al., 2006; Moffett et al., 2007; Pfefferbaum et al., 1999a). Consequently, GM and WM may have different clinical relevance and prognostic value as well as response to treatment. Yet despite nearly 20 years of quantitative in vivo 1H-MRS and extensive literature, the NAA concentration in the GM and WM of the human brain is still debated (Lopez-Villegas et al., 1996; McLean and Barker, 2006; Schuff et al., 2001). These compartmental difference motivated us to develop a method to distinguish the global NAA concentration in WM from GM, presented here. Its main departure from previous 1H-MRS techniques is the trade off of regional localization for global coverage.
While the WM and GM variations in NAA concentration reported in the literature are not necessarily surprising given the myriad acquisition and data analysis approaches employed, brain regions studied, or the cohorts size, age or gender composition, this discordance makes the task of validating a new methodology more difficult. This is because absent an accepted “gold standard” for accuracy, leaves only the precision to be quantified. This is accomplished here under the operational assumptions that in young healthy adults the NAA concentrations of the WM and the GM should be (a) the same and (b) uniform. The ability of a method to reflect this amongst different individuals, given the normal variations in brain volume and its WM/GM fraction, constitutes validation and the variance in this cohort quantifies the technique’s intrinsic precision.
Indeed, the leading findings are that the reproducibility, quantitatively expressed by the coefficient of variations (CV=100% ×mean/SD) is 10.5% in the WM and 7.7% in the GM. This is significantly better than the inter-subject CV≈15% for NAA in localized MRS (Chard et al., 2002; Li et al., 2002). In addition, the average NAA concentration in GM was significantly higher than in WM, in agreement with high resolution in vitro MRS of animal and human normal brain extracts (Florian et al., 1996; Petroff et al., 1989). Unlike in vivo MRS, these in vitro studies can monitor the metabolism of neuronal and glial cells in relative mutual isolation, yielding metabolites’ concentrations from almost “pure” GM and WM. Our findings also agree with many previous in vivo 1H-MRS reports which used multivoxel localization and rigorous segmentation (McLean and Barker, 2006).
Our study offers several improvements over the previous localized 1H-MRS reports. First, the use of the WBNAA sequence eliminates the problem of bone marrow and adipose tissue lipid spectral contamination, facilitating access to the entire cortex. In contrast, previous studies using multislice MRS (even with surface coils that enjoyed good SNR and high special resolution) investigated GM and WM NAA levels in limited brain regions providing important but partial coverage (Lopez-Villegas et al., 1996; Noworolski et al., 1999). Second, the excellent SNR from the large volumes (whole brain and ~0.5 L VOI, see Fig. 1) improved the precision (Li et al., 2002), as reflected by the CVs in Table 1. Third, these two large volumes minimized, due to their smallest surface-to-volume ratio (SVR) the effects of GM/WM/CSF partial-volume segmentation errors at the imaging pixel level to just inter-tissue interfaces. This is compared with localized 1H-MRS where this error is incurred in each and every voxel due to their (much) smaller SVR.
It is also noteworthy that the proposed approach is relatively insensitive to (unknown) T1 and T2 variations between the GM and WM or amongst different brain regions. Specifically, since the WBNAA method employs a very long, TR=10 s, and very short, TE =4 ms, it sustains negligible T1 or T2 weighting (Rigotti et al., 2007). As for the localized MRS component, recent studies have shown that normal regional NAA T2 variations in the human brain lead to less than 10% variations is this metabolite’s quantification (Zaaraoui et al., 2007). As for the dependence on tissue and regional T1s, its has been shown that for a 90° nutation the MR signal changes by less than 5% for ±40% variation (Goelman et al., 2006). Consequently, the proposed approach is insensitive to either normal T1 or T2 variations.
Admittedly, the proposed approach also has several inherent limitations. First, since the concentrations are global averages, (possible) regional variations are lost or ignored even though it is documented that NAA concentrations in some GM, e.g., spinal roots, medulla, pons and neurons involved in local circuits may be substantially lower than in the cortex and in motor neurons (Baslow, 2002; Moffett et al., 2007). Consequently, local changes (if they occur) are maximally diluted, i.e., the approach is insensitive to focal pathology. Given the ~10% CVs, changes must exceed 20% in either compartment in order to achieve statistical significance, although such changes are often encountered in neurological disorders (Inglese et al., 2004; Rigotti et al., 2007). Second, our cohort covered only two decades (20’s through 30’s) of the healthy human lifespan. While this range suits the demographics of some of the common neurological disorders, such as MS, brain trauma and HIV, it does not others, e.g., Alzheimer’s or Parkinson’s diseases. Since it is reasonable to expect that the various aging processes will affect the GM and WM of different individuals differently, it is reasonable to suspect that the precision of the approach may degrade with progressive age for elderly individuals. Finally, the small sample size (N=10) limits the generality of the reported findings and the observed lack of a significant gender difference, therefore, may be a reflection of its statistical power.
Conclusions
High-resolution image segmentation together with whole-brain and localized 1H-MRS can exploit the structural heterogeneity of the brain to yield its average global GM and WM absolute NAA concentration. Since diffuse GM pathology is frequently the primary consequence of neurological diseases and is often the underlying cause of its cognitive sequelae; the possibility of assessing the (entire) cerebral cortex neuronal integrity is particularly attractive for monitoring neuronal changes in both natural history and experimental clinical trials.
Acknowledgment
The authors thank Drs. Andrew A. Maudsley of the University of Miami and Brian J. Soher of Duke University for the use of their SITools-FITT spectral modeling software. This work was supported by NIH grants EB001015, NS0050520 and NS0051623.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold DL, de Stefano N, Matthews PM, Trapp BD. N-acetylaspartate: usefulness as an indicator of viable neuronal tissue. Ann Neurol. 2001;50:823. doi: 10.1002/ana.10013. discussion 824–825. [DOI] [PubMed] [Google Scholar]
- Baslow MH. Evidence supporting a role for N-acetyl-L-aspartate as a molecular water pump in myelinated neurons in the central nervous system. An analytical review. Neurochemistry International. 2002;40:295–300. doi: 10.1016/s0197-0186(01)00095-x. [DOI] [PubMed] [Google Scholar]
- Baslow MH. N-acetylaspartate in the vertebrate brain: metabolism and function. Neurochem Res. 2003;28:941–953. doi: 10.1023/a:1023250721185. [DOI] [PubMed] [Google Scholar]
- Benedetti B, Rigotti DJ, Liu S, Filippi M, Grossman RI, Gonen O. Reproducibility of the Whole-Brain N-Acetylaspartate Level across Institutions, MR Scanners, and Field Strengths. AJNR Am J Neuroradiol. 2007;28:72–75. [PMC free article] [PubMed] [Google Scholar]
- Chard DT, McLean MA, Parker GJ, MacManus DG, Miller DH. Reproducibility of in vivo metabolite quantification with proton magnetic resonance spectroscopic imaging. J Magn Reson Imaging. 2002;15:219–225. doi: 10.1002/jmri.10043. [DOI] [PubMed] [Google Scholar]
- Clark KA, Woods RP, Rottenberg DA, Toga AW, Mazziotta JC. Impact of acquisition protocols and processing streams on tissue segmentation of T1 weighted MR images. Neuroimage. 2006;29:185–202. doi: 10.1016/j.neuroimage.2005.07.035. [DOI] [PubMed] [Google Scholar]
- De Santi S, de Leon MJ, Rusinek H, Convit A, Tarshish CY, Roche A, Tsui WH, Kandil E, Boppana M, Daisley K, Wang GJ, Schlyer D, Fowler J. Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiol Aging. 2001;22:529–539. doi: 10.1016/s0197-4580(01)00230-5. [DOI] [PubMed] [Google Scholar]
- Doyle TJ, Bedell BJ, Narayana PA. Relative concentrations of proton MR visible neurochemicals in gray and white mattter in human brain. Magn Reson Med. 1995;33:755–759. doi: 10.1002/mrm.1910330603. [DOI] [PubMed] [Google Scholar]
- Florian CL, Williams SR, Bhakoo KK, Noble MD. Regional and developmental variations in metabolite concentration in the rat brain and eye: a study using 1H NMR spectroscopy and high performance liquid chromatography. Neurochem Res. 1996;21:1065–1074. doi: 10.1007/BF02532417. [DOI] [PubMed] [Google Scholar]
- Gideon P, Danielsen ER, Schneider M, Henriksen O. Short echo time proton spectroscopy of the brain in healthy volunteers using an insert gradient head coil. Magn Reson Imaging. 1995;13:105–109. doi: 10.1016/0730-725x(94)00073-c. [DOI] [PubMed] [Google Scholar]
- Goelman G, Liu S, Hess D, Gonen O. Optimizing the efficiency of high-field multivoxel spectroscopic imaging by multiplexing in space and time. Magn Reson Med. 2006;56:34–40. doi: 10.1002/mrm.20942. [DOI] [PubMed] [Google Scholar]
- Gonen O, Murdoch JB, Stoyanova R, Goelman G. 3D multivoxel proton spectroscopy of human brain using a hybrid of 8th-order Hadamard encoding with 2D chemical shift imaging. Magn Reson Med. 1998a;39:34–40. doi: 10.1002/mrm.1910390108. [DOI] [PubMed] [Google Scholar]
- Gonen O, Viswanathan AK, Catalaa I, Babb J, Udupa J, Grossman RI. Total brain N-acetylaspartate concentration in normal, age-grouped females: quantitation with non-echo proton NMR spectroscopy. Magn Reson Med. 1998b;40:684–689. doi: 10.1002/mrm.1910400506. [DOI] [PubMed] [Google Scholar]
- Hetherington HP, Pan JW, Mason GF, Adams D, Vaughn MJ, Twieg DB, Pohost GM. Quantitative 1H spectroscopic imaging of human brain at 4.1 T using image segmentation. Magn Reson Med. 1996;36:21–29. doi: 10.1002/mrm.1910360106. [DOI] [PubMed] [Google Scholar]
- Inglese M, Ge Y, Filippi M, Falini A, Grossman RI, Gonen O. Indirect evidence for early widespread gray matter involvement in relapsing-remitting multiple sclerosis. Neuroimage. 2004;21:1825–1829. doi: 10.1016/j.neuroimage.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Inglese M, Li BS, Rusinek H, Babb JS, Grossman RI, Gonen O. Diffusely elevated cerebral choline and creatine in relapsing-remitting multiple sclerosis. Magn Reson Med. 2003;50:190–195. doi: 10.1002/mrm.10481. [DOI] [PubMed] [Google Scholar]
- Jansen JF, Backes WH, Nicolay K, Kooi ME. 1H MR spectroscopy of the brain: absolute quantification of metabolites. Radiology. 2006;240:318–332. doi: 10.1148/radiol.2402050314. [DOI] [PubMed] [Google Scholar]
- Kingsley PB, Shah TC, Woldenberg R. Identification of diffuse and focal brain lesions by clinical magnetic resonance spectroscopy. NMR Biomed. 2006;19:435–462. doi: 10.1002/nbm.1039. [DOI] [PubMed] [Google Scholar]
- Kreis R, Ernst T, Ross BD. Absolute quantitation of water and metabolites in the human Brain. II. Metabolite Concentrat ions. J Magn Reson. Series B. 1993;102:9–19. [Google Scholar]
- Li BS, Babb JS, Soher BJ, Maudsley AA, Gonen O. Reproducibility of 3D proton spectroscopy in the human brain. Magn Reson Med. 2002;47:439–446. doi: 10.1002/mrm.10081. [DOI] [PubMed] [Google Scholar]
- Lim KO, Spielman DM. Estimating NAA in cortical gray matter with applications for measuring changes due to aging. Magn Reson Med. 1997;37:372–377. doi: 10.1002/mrm.1910370313. [DOI] [PubMed] [Google Scholar]
- Lopez-Villegas D, Kimura H, Tunlayadechanont S, Lenkinski RE. High spatial resolution MRI and proton MRS of human frontal cortex. NMR Biomed. 1996;9:297–304. doi: 10.1002/(SICI)1099-1492(199610)9:7<297::AID-NBM433>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- McLean MA, Barker GJ. Concentrations and magnetization transfer ratios of metabolites in gray and white matter. Magn Reson Med. 2006;56:1365–1370. doi: 10.1002/mrm.21070. [DOI] [PubMed] [Google Scholar]
- Michaelis T, Merboldt KD, Bruhn H, Hanicke W, Frahm J. Absolute concentrations of metabolites in the adult human brain in vivo: quantification of localized proton MR spectra. Radiology. 1993;187:219–227. doi: 10.1148/radiology.187.1.8451417. [DOI] [PubMed] [Google Scholar]
- Mikheev A, Nevsky G, Govindan S, Grossman RI, Rusinek H. Fully automatic segmentation of the brain from T1-weighted MRI using Bridge Burner algorithm. J. Magn. Reson Imag. 2008 doi: 10.1002/jmri.21372. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonen CTW, Sobering G, van Zijl PCM, Gillen J, von Kienlin M, Bizzi A. Proton spectroscopic imaging of human brain. J. Magn. Reson. 1992;98:556–575. [Google Scholar]
- Narayana PA, Fotedar LK, Jackson EF, Bohan TP, Butler IJ, Wolinsky JS. Regional in vivo proton magnetic resonance spectroscopy of brain. J Magn Reson. 1989;83:44–52. [Google Scholar]
- Noworolski SM, Nelson SJ, Henry RG, Day MR, Wald LL, Star-Lack J, Vigneron DB. High spatial resolution 1H-MRSI and segmented MRI of cortical gray matter and subcortical white matter in three regions of the human brain. Magn Reson Med. 1999;41:21–29. doi: 10.1002/(sici)1522-2594(199901)41:1<21::aid-mrm5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Petroff OA, Spencer DD, Alger JR, Prichard JW. High-field proton magnetic resonance spectroscopy of human cerebrum obtained during surgery for epilepsy. Neurology. 1989;39:1197–1202. doi: 10.1212/wnl.39.9.1197. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Spielman D, Sullivan EV, Lim KO. In vivo brain concentrations of N-acetyl compounds, creatine, and choline in Alzheimer disease. Arch Gen Psychiatry. 1999a;56:185–192. doi: 10.1001/archpsyc.56.2.185. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Spielman D, Sullivan EV, Lim KO. In vivo spectroscopic quantification of the N-acetyl moiety, creatine, and choline from large volumes of brain gray and white matter: effects of normal aging. Magn Reson Med. 1999b;41:276–284. doi: 10.1002/(sici)1522-2594(199902)41:2<276::aid-mrm10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Rigotti DJ, Inglese M, Gonen O. Whole-brain N-acetylaspartate as a surrogate marker of neuronal damage in diffuse neurologic disorders. AJNR Am J Neuroradiol. 2007;28:1843–1849. doi: 10.3174/ajnr.A0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross BD, Bluml S. Magnetic resonance Spectroscopy of the human brain. Anat Rec. 2001;265:54–84. doi: 10.1002/ar.1058. [DOI] [PubMed] [Google Scholar]
- Rusinek H, Chandra R. Brain tissue volume measurement from magnetic resonance imaging. A phantom study. Invest Radiol. 1993;28:890–895. doi: 10.1097/00004424-199310000-00004. [DOI] [PubMed] [Google Scholar]
- Schuff N, Ezekiel F, Gamst AC, Amend DL, Capizzano AA, Maudsley AA, Weiner MW. Region and tissue differences of metabolites in normally aged brain using multislice 1H magnetic resonance spectroscopic imaging. Magn Reson Med. 2001;45:899–907. doi: 10.1002/mrm.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuffq N, Meyerhoff DJ, Mueller S, Chao L, Sacrey DT, Laxer K, Weiner MW. N-acetylaspartate as a marker of neuronal injury in neurodegenerative disease. Adv Exp Med Biol. 2006;576:241–262. doi: 10.1007/0-387-30172-0_17. discussion 361–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons ML, Frondoza CG, Coyle JT. Immunocytochemical localization of N-acetyl-aspartate with monoclonal antibodies. Neuroscience. 1991;45:37–45. doi: 10.1016/0306-4522(91)90101-s. [DOI] [PubMed] [Google Scholar]
- Soher BJ, van Zijl PC, Duyn JH, Barker PB. Quantitative proton MR spectroscopic imaging of the human brain. Magn Reson Med. 1996;35:356–363. doi: 10.1002/mrm.1910350313. [DOI] [PubMed] [Google Scholar]
- Soher BJ, Young K, Govindaraju V, Maudsley AA. Automated spectral analysis III: application to in vivo proton MR spectroscopy and spectroscopic imaging. Magn Reson Med. 1998;40:822–831. doi: 10.1002/mrm.1910400607. [DOI] [PubMed] [Google Scholar]
- Stadlbauer A, Moser E, Gruber S, Buslei R, Nimsky C, Fahlbusch R, Ganslandt O. Improved delineation of brain tumors: an automated method for segmentation based on pathologic changes of 1H-MRSI metabolites in gliomas. Neuroimage. 2004;23:454–461. doi: 10.1016/j.neuroimage.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Tallan HH, Moore S, Stein WH. N-acetyl-L-aspartic acid in brain. J. Biol. Chem. 1956;219:257–264. [PubMed] [Google Scholar]
- Tedeschi G, Righini A, Bizzi A, Barnett AS, Alger JR. Cerebral white matter in the centrum semiovale exhibits a larger N-acetyl signal than does gray matter in long echo time 1H-magnetic resonance spectroscopic imaging. Magn Reson Med. 1995;33:127–133. doi: 10.1002/mrm.1910330120. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li SJ. Differentiation of metabolic concentrations between gray matter and white matter of human brain by in vivo 1H magnetic resonance spectroscopy. Magn Reson Med. 1998;39:28–33. doi: 10.1002/mrm.1910390107. [DOI] [PubMed] [Google Scholar]
- Weber-Fahr W, Ende G, Braus DF, Bachert P, Soher BJ, Henn FA, Buchel C. A fully automated method for tissue segmentation and CSF-correction of proton MRSI metabolites corroborates abnormal hippocampal NAA in schizophrenia. Neuroimage. 2002;16:49–60. doi: 10.1006/nimg.2002.1057. [DOI] [PubMed] [Google Scholar]
- Zaaraoui W, Fleysher L, Fleysher R, Liu S, Soher BJ, Gonen O. Human brain-structure resolved T(2) relaxation times of proton metabolites at 3 Tesla. Magn Reson Med. 2007;57:983–989. doi: 10.1002/mrm.21250. [DOI] [PubMed] [Google Scholar]


