Abstract
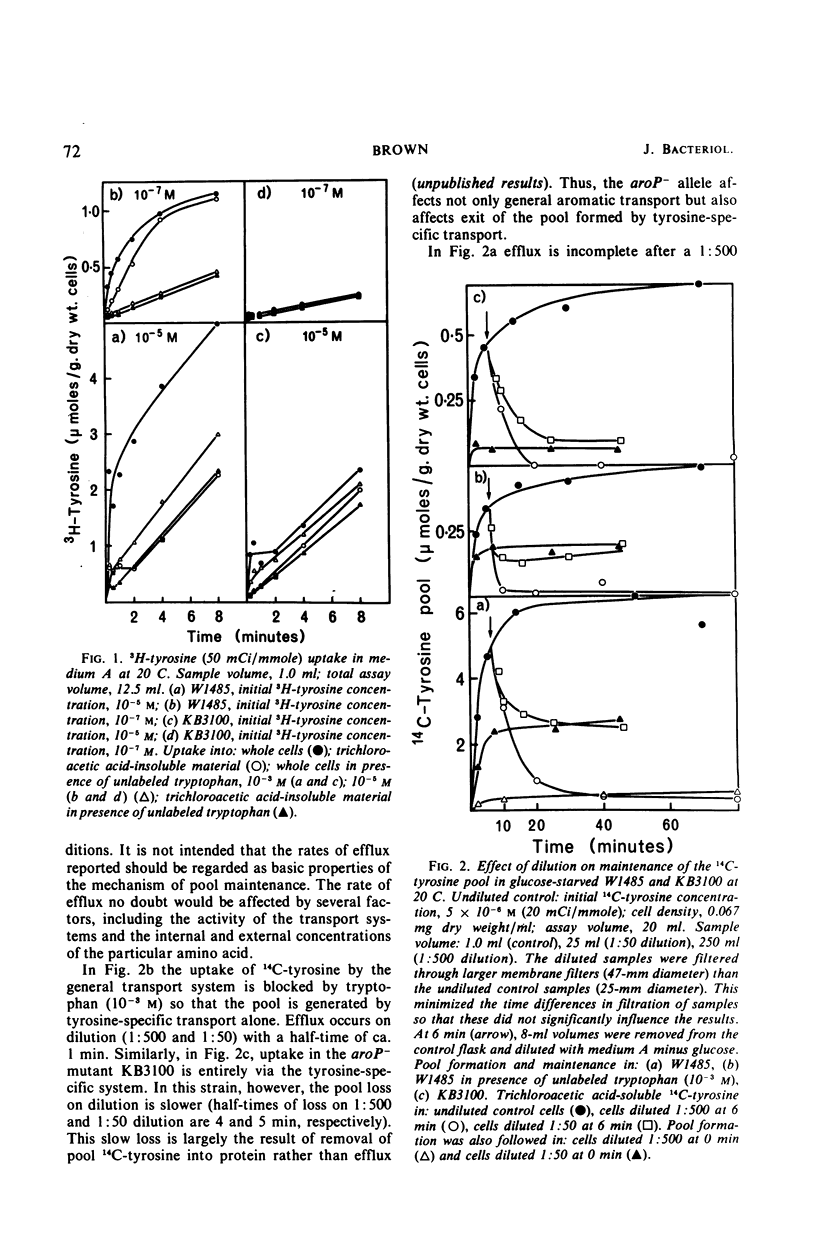
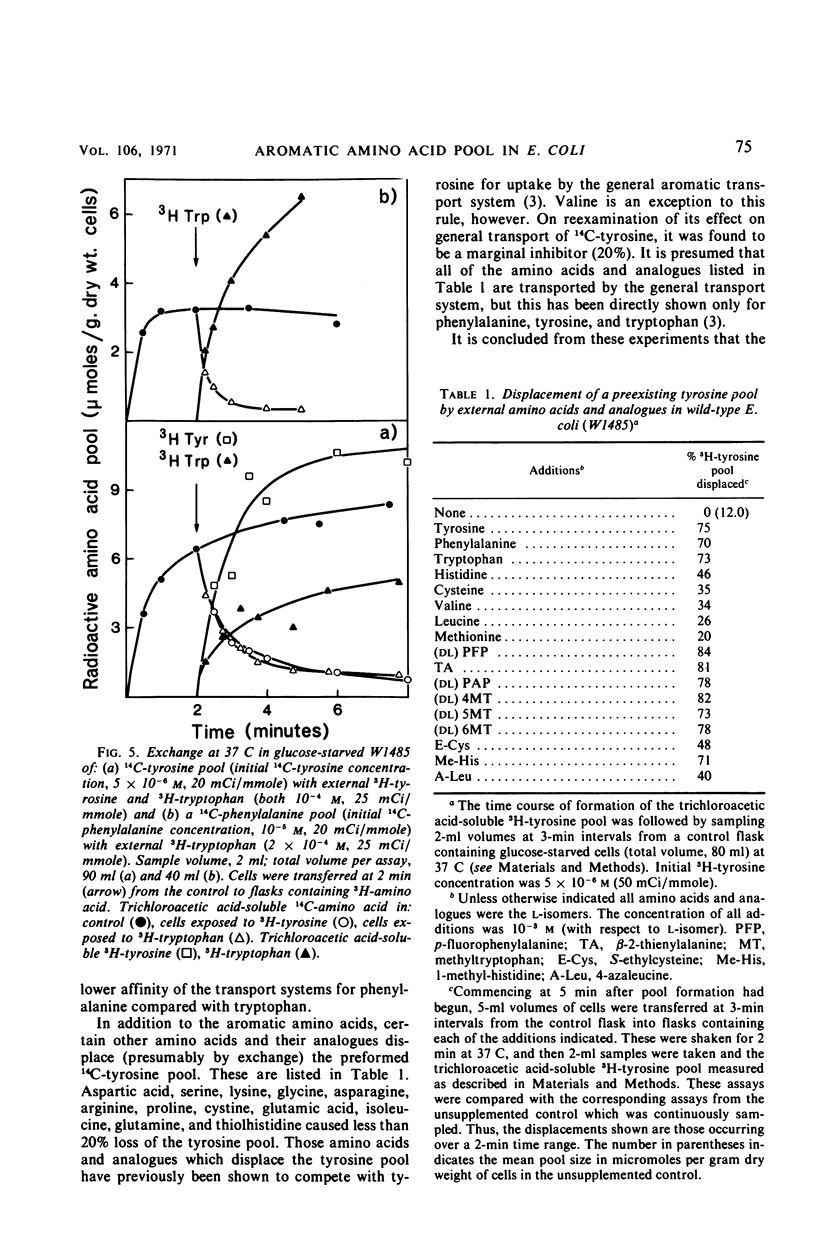
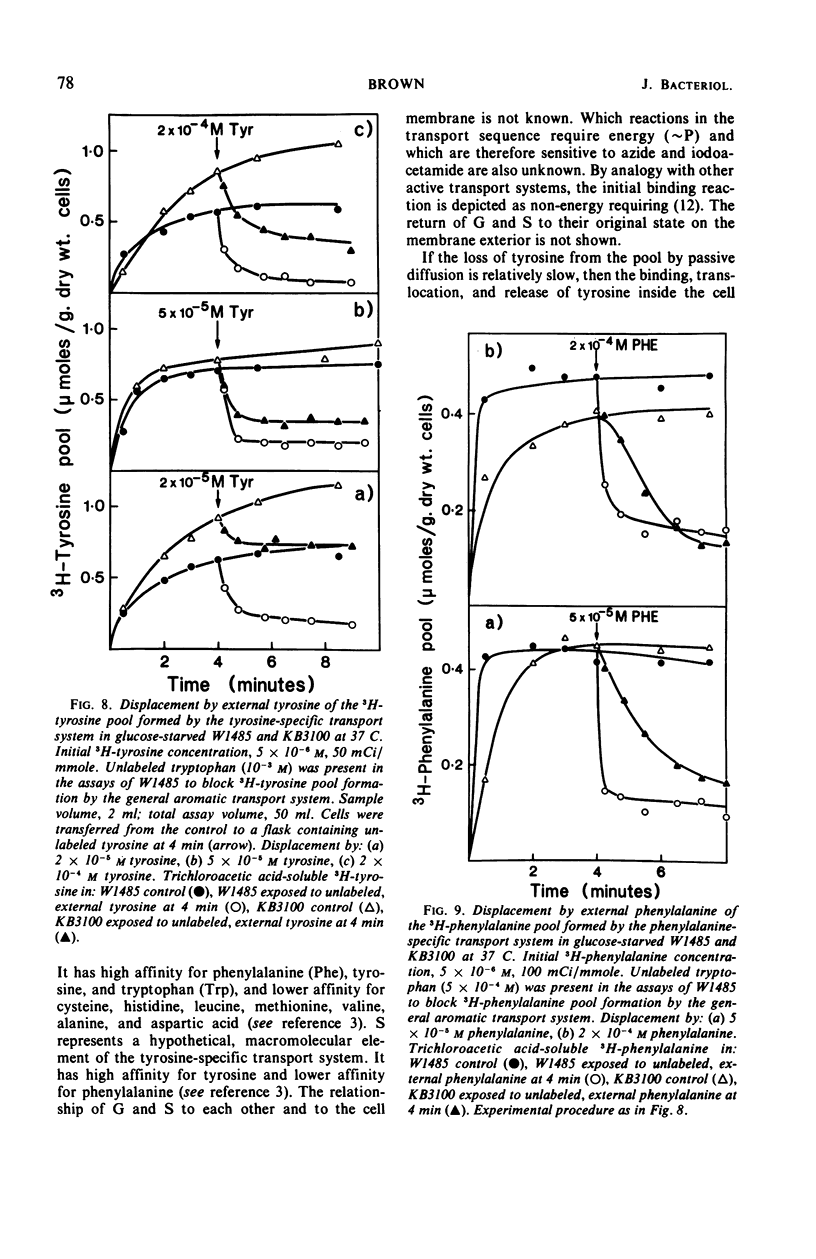
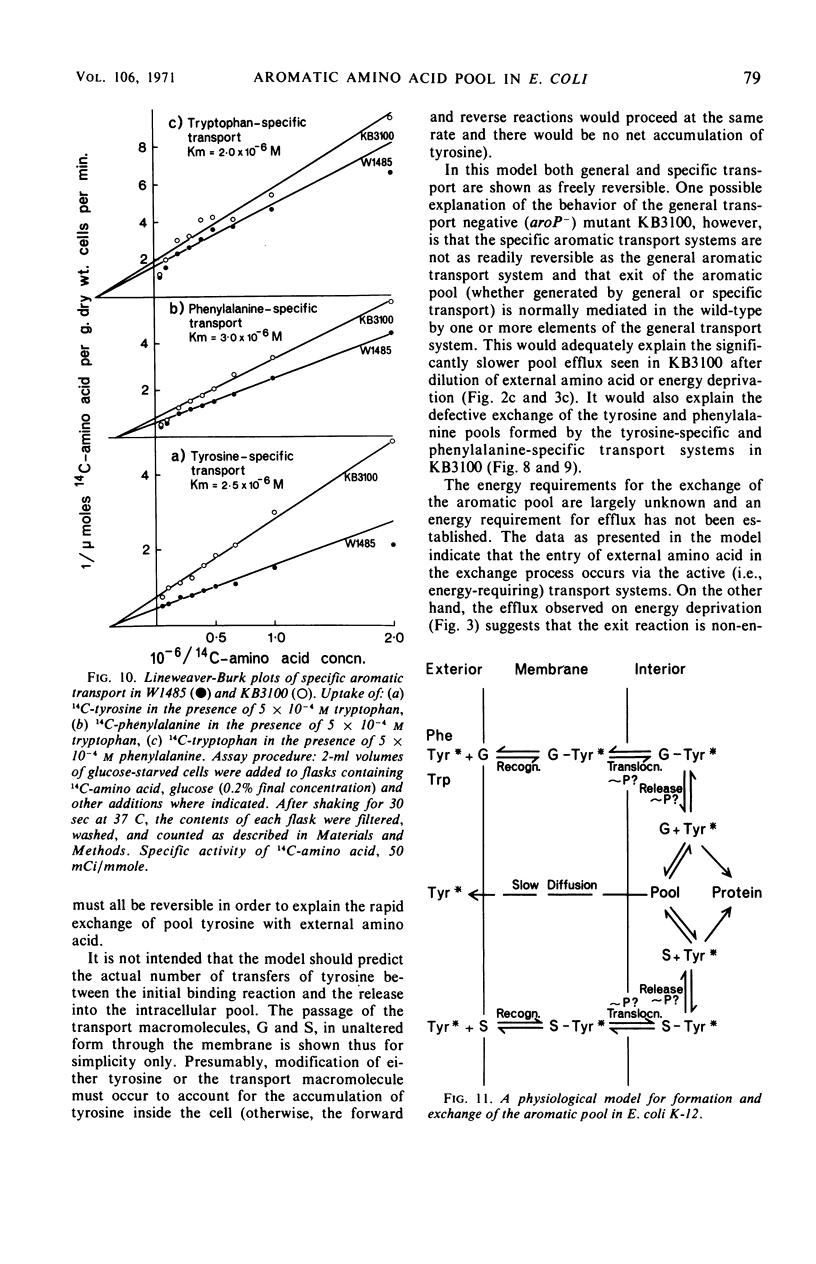
The pool of phenylalanine, tyrosine, and tryptophan is formed in Escherichia coli K-12 by a general aromatic transport system [Michaelis constant (Km) for each amino acid approximately 5 × 10−7m] and three further transport systems each specific for a single aromatic amino acid (Km for each amino acid approximately 2 × 10−6m, reference 3). When the external concentration of a particular aromatic amino acid is saturating for both classes of transport system, the free amino acid pool is supplied with external amino acid by both systems. Blocking the general transport system reduces the pool size by 80 to 90% but does not interfere with the supply of the amino acid to protein synthesis. If, however, the external concentration is too low to saturate specific transport, blocking general transport inhibits the incorporation of external amino acid into protein by about 75%. It is concluded that the amino acids transported by either class of transport system can be used for protein synthesis. Dilution of the external amino acid or deprivation of energy causes efflux of the aromatic pool. These results and rapid exchange observed between pool amino acid and external amino acids indicate that the aromatic pool circulates rapidly between the inside and the outside of the cell. Evidence is presented that this exchange is mediated by the aromatic transport systems. Mutation of aroP (a gene specifying general aromatic transport) inhibits exit and exchange of the small pool generated by specific transport. These findings are discussed and a simple physiological model of aromatic pool formation, and exchange, is proposed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES G. F. UPTAKE OF AMINO ACIDS BY SALMONELLA TYPHIMURIUM. Arch Biochem Biophys. 1964 Jan;104:1–18. doi: 10.1016/s0003-9861(64)80028-x. [DOI] [PubMed] [Google Scholar]
- BRITTEN R. J., McCLURE F. T. The amino acid pool in Escherichia coli. Bacteriol Rev. 1962 Sep;26:292–335. doi: 10.1128/br.26.3.292-335.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D. Formation of aromatic amino acid pools in Escherichia coli K-12. J Bacteriol. 1970 Oct;104(1):177–188. doi: 10.1128/jb.104.1.177-188.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D. Regulation of aromatic amino acid biosynthesis Escherichia coli K12. Genetics. 1968 Sep;60(1):31–48. doi: 10.1093/genetics/60.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBusk B. G., DeBusk A. G. Molecular transport in Neurospora crassa. I. Biochemical properties of a phenylalanine permease. Biochim Biophys Acta. 1965 Jun 15;104(1):139–150. doi: 10.1016/0304-4165(65)90229-1. [DOI] [PubMed] [Google Scholar]
- Grivell A. R., Jackson J. F. Microbial culture preservation with silica gel. J Gen Microbiol. 1969 Nov;58(3):423–425. doi: 10.1099/00221287-58-3-423. [DOI] [PubMed] [Google Scholar]
- KESSEL D., LUBIN M. Transport of proline in Escherichia coli. Biochim Biophys Acta. 1962 Feb 12;57:32–43. doi: 10.1016/0006-3002(62)91074-0. [DOI] [PubMed] [Google Scholar]
- Kay W. W., Gronlund A. F. Amino acid pool formation in Pseudomonas aeruginosa. J Bacteriol. 1969 Jan;97(1):282–291. doi: 10.1128/jb.97.1.282-291.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B. Membrane transport proteins. Proteins that appear to be parts of membrane transport systems are being isolated and characterized. Science. 1968 Nov 8;162(3854):632–637. doi: 10.1126/science.162.3854.632. [DOI] [PubMed] [Google Scholar]


