Abstract
The interaction of sodium and potassium ions in the context of the primary entry of Na+ into plant cells, and the subsequent development of sodium toxicity, has been the subject of much recent attention. In the present study, the technique of compartmental analysis with the radiotracers 42K+ and 24Na+ was applied in intact seedlings of barley (Hordeum vulgare L.) to test the hypothesis that elevated levels of K+ in the growth medium will reduce both rapid, futile Na+ cycling at the plasma membrane, and Na+ build-up in the cytosol of root cells, under saline conditions (100 mM NaCl). We reject this hypothesis, showing that, over a wide (400-fold) range of K+ supply, K+ neither reduces the primary fluxes of Na+ at the root plasma membrane nor suppresses Na+ accumulation in the cytosol. By contrast, 100 mM NaCl suppressed the cytosolic K+ pool by 47–73%, and also substantially decreased low-affinity K+ transport across the plasma membrane. We confirm that the cytosolic [K+]:[Na+] ratio is a poor predictor of growth performance under saline conditions, while a good correlation is seen between growth and the tissue ratios of the two ions. The data provide insight into the mechanisms that mediate the toxic influx of sodium across the root plasma membrane under salinity stress, demonstrating that, in the glycophyte barley, K+ and Na+ are unlikely to share a common low-affinity pathway for entry into the plant cell.
Keywords: Barley, compartmental analysis, cytosol, influx, efflux, potassium, radiotracers, salinity, salt stress, sodium
Introduction
Increasing salinization of agricultural soils is one of the most challenging issues faced by modern agriculture. In excess of 30% of cultivated soils are affected by salinity (Epstein et al., 1980; Zhu et al., 1997; Zhu, 2001; Munns, 2005). Much of this salinization is attributable to the infiltration and accumulation of NaCl (Zhu, 2001; Munns, 2005), often resulting in soil Na+ concentrations above 40 mM, and growth suppression in most crops (Munns, 2005). One of the key physiological processes disrupted by Na+ supply in this toxic range is the maintenance of cellular and whole-plant potassium homeostasis (Rains and Epstein, 1967; Flowers and Läuchli, 1983; Watad et al., 1991; Gaxiola et al., 1992; Warne et al., 1996; Zhu et al., 1998; Santa-María and Epstein, 2001; Peng et al., 2004; Cakmak, 2005; Kader and Lindberg, 2005; Kronzucker et al., 2006; Takahashi et al., 2007). At the tissue level, the ratio of K+ to Na+ is considered an excellent indicator of plant tolerance to salinity; the higher the ratio, the higher the plant's tolerance (Flowers and Hajibagheri, 2001; Cakmak, 2005; Chen et al., 2007b; cf. Genc et al., 2007). As a result of this observation, selection or breeding cultivars that maintain high K+:Na+ ratios has emerged as an important strategy to counteract the detrimental effects of soil salinity (Deal et al., 1999; Santa-María and Epstein, 2001). A more precise proposal has been that a high K+:Na+ ratio specifically in the cytosolic compartment is critical to plant survival under sodium challenge, while a decrease in this ratio will predict the onset of salinity stress and growth decline (Hajibagheri et al., 1987, 1989; Maathuis and Amtmann, 1999; Flowers and Hajibagheri, 2001; Carden et al., 2003; Peng et al., 2004; Kader et al., 2006; James et al., 2006; Chen et al., 2007a; Davenport et al., 2007; Obata et al., 2007; Takahashi et al., 2007). This proposal has gained wide acceptance, even though cytosolic K+:Na+ ratios are, in fact, rarely measured.
In a recent study in barley (Hordeum vulgare L.), it was shown that, at low to intermediate levels of external K+ supply ([K+]ext=0.1–1.5 mM), and at varying salinity levels, the ratio did not in fact correlate with seedling growth in this major cereal (Kronzucker et al., 2006). On the contrary, no difference in growth was observed in the presence of a >5-fold variation in the cytosolic K+:Na+ ratio. The study further demonstrated that Na+ suppressed K+ influx across the plasma membrane to a similar extent at 0.1 and 1.5 mM [K+]ext, concentrations at which high-affinity transport systems for K+ predominate (Epstein et al., 1963; Kochian and Lucas, 1982; Szczerba et al., 2006), while Na+ influxes and cytosolic pools were unaffected by K+. However, a significant suppression of the cytosolic K+ pool by Na+ was seen only at the higher [K+]ext, suggesting that different cellular responses may come into effect as high-affinity K+ transport gives way to low-affinity transport (see Szczerba et al., 2006).
In the present study, to examine further the proposed pivotal role of K+ homeostasis in salinity stress and tolerance, the effects of K+ supply across the low-affinity transport range of K+ (up to 40 mM [K+]ext) upon the primary fluxes and cytosolic pools of Na+, and, conversely, the effects of Na+ upon K+ fluxes and pools in this range were investigated. Such an examination was particularly necessary in the light of recent disagreements in the literature pertaining to (i) the size of cytosolic Na+ pools (Flowers and Hajibagheri, 2001; Carden et al., 2003; James et al., 2006; Kronzucker et al., 2006), and (ii) the proposed, but as yet unresolved, roles of molecular candidates for toxic Na+ influx into the plant (Tester and Davenport, 2003; Flowers, 2006; Wang et al., 2007). The primary candidates proposed are K+-specific channels (Wang et al., 2007), non-selective cation channels (NSCCs; Demidchik et al., 2002), HKT-type transporters (Rodriguez-Navarro and Rubio, 2006), and the low-affinity cation transporter LCT (Amtmann et al., 2001). Indeed, were these candidates to catalyse toxicologically significant fluxes of sodium, they should be competitively influenced by the presence of potassium. For these reasons, a detailed study of the interactions between the two ions was carried out along a wide gradient of external K+ supply.
Materials and methods
Plant growth
Seeds of barley (Hordeum vulgare L. cv. Klondike) were surface-sterilized by immersing seeds in 1.0% sodium hypochlorite for 10 min. Seeds were then washed under running tap water for 3 h, placed on discs of plastic mesh, and covered by 2 cm of moist sand. Germination proceeded for the following 3 d in a walk-in growth chamber equipped with fluorescent lights (Philips Econ-o-watt, F96T12, with an irradiation of 200 μmol photons m−2 s−1 at plant height, for 16 h d−1), and having a day/night temperature cycle of 20 °C/15 °C, and relative humidity of ∼70%.
Following germination, seedlings were transferred to opaque plastic 4 l hydroponic vessels, filled with modified quarter-strength Johnson's solution, consisting of: 5 mM Ca(NO3)2, 0.5 mM NaH2PO4, 0.25 mM MgSO4, 0.2 mM CaSO4, 0.125 μM Na2MoO4, 20 μM FeEDTA, 25 μM H3BO3, 2 μM ZnSO4, 0.5 μM MnSO4, and 0.5 μM CuSO4. Control plants had no additional sodium, while salt-stressed plants were treated with 100 mM Na+ (as NaCl). Potassium concentrations were adjusted to treatments of 1.5, 5, 10, 20, and 40 mM by addition of K2SO4. pH was adjusted to 6.3–6.5 by addition of NaOH. To prevent nutrient depletion, solutions were replaced after 2 d. Plants remained in hydroponic solutions for 4 d prior to experimentation. In select treatments (1.5 mM and 40 mM K+, at low and high NaCl), plants were also grown for 2 weeks (11 d in solution; see Fig. 9B).
Fig. 9.
(A) Fresh weights of barley seedlings (roots+shoots), grown and monitored with or without 100 mM Na+ and varying levels of K+ supply. Error bars refer to ±SEM of 12–72 replicates. Asterisks denote significant differences within a given K+ treatment (P < 0.05). (B) Fresh weights of barley seedlings (roots+shoots), grown and monitored with or without 100 mM Na+ and two different [K+]ext (1.5 mM and 40 mM) for 2 weeks. Error bars refer to ±SEM of 5–82 replicates.
Flux analysis
Compartmental analysis by tracer efflux was conducted as described in detail elsewhere (Kronzucker et al., 1999, 2006; Britto et al., 2001, 2006). In brief, roots of intact seedlings were immersed for 1 h in a nutrient solution identical to the growth solution, except that it contained 24Na+ or 42K+ in addition to non-radioactive Na+ or K+. Roots were desorbed of radioactivity in tracer-free solutions for the monitoring of 24Na+ or 42K+ efflux, by periodic washing with a timed series of non-radioactive aliquots of nutrient solution (Kronzucker et al., 1999, 2006; Britto et al., 2001). The time course of aliquots was as follows: 15 s (4×), 20 s (3×), 30 s (2×), 40 s (1×), 50 s (1×), 1 min (5×), 1.25 min (1×), 1.5 min (1×), 1.75 min (1×), and 2 min (8×). Radioactivity was measured in aliquots and plant tissues by gamma counting, using a Canberra-Packard counter, Quantum Cobra Series II, Model 5003.
Unidirectional Na+ or K+ fluxes were determined using standard analyses (for further details, see Kronzucker et al., 1999, 2003, 2006; Britto et al., 2001):
(i) Efflux (ϕco) was calculated from the initial rate of 24Na+ or 42K+ release from the cytosol, divided by initial cytosolic specific activity (Sc) of 24Na+ or 42K+ in this compartment; Sc was estimated by using labelling time (tL) in the external medium of known specific activity (So), and the kinetic constant k that describes the exponential rate of cytosolic tracer exchange, using the relationship Sc=So(1–e–kt). This constant was determined from the slope of the cytosolic line (see Fig. 1).
(ii) Net fluxes were determined from retention of tracer in root and shoot at the end of the desorption protocol, divided by So, while influx (ϕoc) was calculated from the sum of ϕco and the net flux.
(iii) Cytosolic concentrations of Na+ ([Na+]cyt) or K+ ([K+]cyt) were determined by integrating rates of radioactivity release from this compartment; in simplified form, this calculation is made using the equation [Na+ or K+]cyt=Ωϕoc/k, where Ω is a proportionality constant accounting for the cytosolic compartment comprising 5% of tissue volume. Activity coefficients (γ) for cytosolic concentrations were estimated using the Debye–Hückel–Onsager equation, adapted for the monovalent cations Na+ and K+: –logγ=(0.5√I)/(1+√I) where I is ionic strength of the cytosol (assuming that the dominant cations are Na+ and K+, and that these are charge-balanced by monovalent anions; see Jander and Blasius, 1988).
Fig. 1.
Representative plots of 42K+ and 24Na+ (inset) efflux from roots of intact barley seedlings, under varying ionic conditions. Roots had been preloaded for 60 min in radioactive solution, then eluted of radioactivity in a timed series of non-radioactive growth-solution aliquots. Dashed lines represent the slowest exchanging compartment (cytosolic), with a minimum of 12 time points used for linear regression. Experiments were replicated between 4 and 14 times.
Tissue K+ and Na+ content
Roots of barley seedlings were desorbed for 5 min in 10 mM CaSO4 to remove extracellular K+ and Na+. Roots and shoots were then separated, weighed, and oven dried for a minimum of 72 h at 80–85 °C, then pulverized and digested with 30% HNO3 for a minimum of 72 h. K+ and Na+ concentration was determined using a single-channel flame photometer (Digital Flame Analyzer model 2655-00; Cole-Parmer, Anjou, Québec).
Statistical analysis
Statistical analyses were conducted using one-way analysis of variance (ANOVA) with the statistical package SPSS (ver. 12).
Results and discussion
Figure 1 shows representative plots of 42K+ (main plot) and 24Na+ (inset) release from roots of intact barley seedlings, previously labelled with respective tracers for 60 min. Steady-state tracer efflux curves of this type were analysed under five external potassium conditions (1.5, 5, 10, 20, and 40 mM) in control (1 mM NaCl) or salt-treated (100 mM NaCl) plants, to determine unidirectional fluxes of Na+ and K+ across the plasma membrane of root cells, kinetic constants for cytosolic exchange of the two ions, and estimates of the ions' cytosolic activities. In Fig. 1, the main plot shows the potent reduction, by elevated Na+ provision, of the efflux of K+ from barley roots (note that the y-axis is logarithmic), a phenomenon paralleled in reductions in the influx and net flux of K+ (see Fig. 2). By contrast, the inset of this figure shows the lack of a reciprocal effect: a 27-fold difference in K+ supply had no influence on the efflux of Na+ under saline conditions.
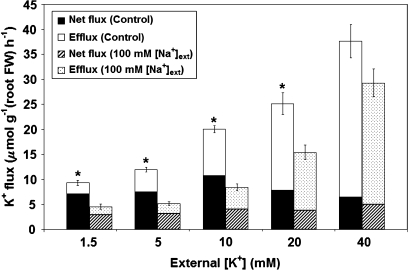
Fig. 2.
Components of unidirectional K+ influx in roots of intact barley seedlings, grown and monitored with or without 100 mM Na+. Control values are drawn from Szczerba et al. (2006). Error bars refer to ±SEM of 4–14 replicates, with asterisks indicating significant differences in influx values within a given K+ treatment (P < 0.05). Where differences in influx between Na+ treatments were observed, significant differences in efflux and net flux were also found (P < 0.05).
Figure 2 shows the steady-state unidirectional and net fluxes of K+ into barley roots, obtained using compartmental tracer analysis under the 10 conditions examined. Over the range of K+ supply, the influx of potassium in salt-treated plants was lower than in controls (by 20–60%), significantly so in all cases except for 40 mM [K+]ext (P < 0.05). Work by Kochian et al. (1985) showed that application of 3 mM Na+ resulted in a 50% suppression of K+ influx in maize, but this effect was limited to the low-affinity transport range for K+, and was only seen in ‘low-salt’ plants. This result contrasts with the present study and with previous work in barley, which showed the suppression, by Na+, of K+ influx at the high-affinity supply provision of 0.1 mM (Kronzucker et al., 2006), as well as in the low-affinity range in the ‘high-salt’ plants (grown at up to 40 mM K+); two possible explanations for this disagreement include the use of a much higher Na+ provision (100 mM), and the examination of plants grown and tested under steady-state nutritional conditions. Consistent with the present work, effects of Na+ on K+ uptake have also been observed in both high- and low-affinity transport ranges in a wide range of other studies (Rains and Epstein, 1967; Santa-María et al., 1997; Fu and Luan, 1998; Rubio et al., 2000, 2003; Martínez-Cordero et al., 2005).
Net fluxes and unidirectional effluxes of K+ were also reduced under elevated NaCl, significantly so in all cases except for the 40 mM K+ treatment (Fig. 2). The reduction of K+ efflux with high NaCl provision (Figs 1, 2) may appear, on the surface, to contradict the finding that Na+ stimulated a net efflux of K+ from roots of several plant species (see, for instance, Chen et al., 2005; Cuin and Shabala, 2007), but it must be pointed out that such demonstrations of Na+-stimulated K+ efflux involve short-term changes following NaCl shock, whereas the present study involves measurements made under steady-state conditions.
Influx and efflux of Na+ at 100 mM [Na+]ext were high under all K+ conditions (Fig. 3), and indicated substantial futile cycling of the ion at the plasma membrane. Rapid unidirectional influxes of Na+ in glycophytes under salinity conditions have been observed by others (Essah et al., 2003; Wang et al., 2006; Davenport et al., 2007; Horie et al. 2007); in particular, the influx values reported here are in excellent agreement with short-term 22Na+ labelling data in a recent study by Chen et al. (2007a) that examined Na+ influx in four cultivars of barley. The high ratios of efflux to influx seen consistently throughout the present treatments are also supported by previous studies (Cheeseman, 1982; Mills et al., 1985; Essah et al., 2003). In sharp contrast to the suppression of unidirectional and net K+ fluxes by Na+, a reciprocal influence was not seen. At 100 mM [Na+]ext, the 27-fold variation in K+ supply resulted in no significant differences in influx, efflux, or net flux of sodium. Previous work on the interactions between these ions in the high-affinity K+ transport range (Kronzucker et al., 2006) also showed no significant differences in Na+ between 0.1 mM and 1.5 mM external [K+]. The two studies together therefore show that a 400-fold range of K+ provision fails to modify Na+ fluxes, while K+ fluxes across this range are generally suppressed by elevated sodium. This lack of reciprocity was further confirmed by experiments conducted in 2-week-old seedlings of barley, indicating that the pattern was not limited to one developmental stage (data not shown). This conclusion, however, was not drawn in a classic study, also with barley, by Rains and Epstein (1967): a strong reciprocal suppression of the flux of one ion (K+ or Na+) by the co-presence of the other was observed in the low-affinity ‘mechanism 2 of alkali cation transport’. Again, however, it is important to note that the present study differed from that of Rains and Epstein (1967) in that the fluxes in the present study were measured under steady-state nutritional supply conditions, which may be more relevant to plant performance in the field than perturbational conditions might be. In a recent study, Wang et al. (2007) also showed that K+ supply had no effect on Na+ uptake in the halophyte Suaeda maritima, but only when the external [Na+] was below 75 mM. Above that concentration, a suppression of Na+ fluxes was found, indicating, in Suaeda, the involvement of AKT-type potassium channels in Na+ influx under some conditions.
Fig. 3.
Components of unidirectional Na+ influx in roots of intact barley seedlings, grown and monitored at 100 mM Na+ and at varying external K+ provision. Error bars refer to ±SEM of four to seven replicates. No significant differences between treatments were observed.
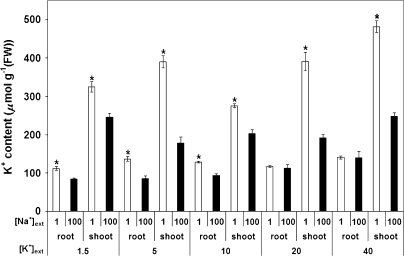
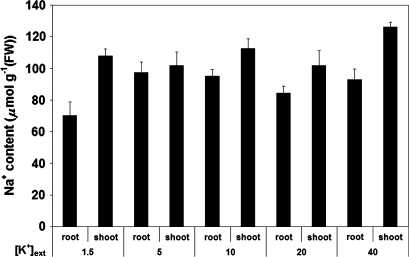
In the present study, the steady-state, non-reciprocal effect of one ion on the fluxes of the other is borne out in the tissue accumulation patterns for K+ and Na+ (Figs 4, 5). For potassium, there was a general reduction of tissue content with salt treatment, particularly in the shoot, where tissue K+ levels were seen to decline by as much as 50% (Fig. 4). The contrasting situation for sodium is seen in Fig. 5; as with Na+ fluxes, the accumulation of this ion was not changed over the range of applied external [K+], either in the root or in the shoot.
Fig. 4.
Tissue content of K+ in roots and shoots of barley plants, grown with or without 100 mM Na+, and under varying external K+ provision. Control (1 mM Na+) values are drawn from Szczerba et al. (2006). Error bars refer to ±SEM of four to eight replicates. Asterisks denote significant differences within a given K+ treatment and plant organ (P < 0.05).
Fig. 5.
Tissue content of Na+ in roots and shoots of barley plants, grown at 100 mM Na+, and under varying external K+ provision. Error bars refer to ±SEM of 8–12 replicates. No significant differences between treatments were observed.
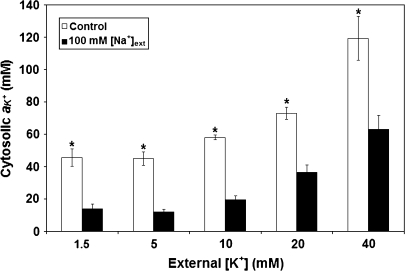
On a finer scale of analysis, a steady-state, non-reciprocal interaction was also seen in the effects of ion supply on the activities of Na+ and K+ in the cytosolic compartment of root cells (aNa+,cyt and aK+,cyt; Figs 6, 7), as estimated by compartmental analysis. Under salinity conditions, aNa+,cyt proved to be resistant to variations in K+ provision, showing no significant changes across the entire range of external K+ supply (Fig. 7). By comparison, 100 mM sodium provision resulted in severe drops in aK+,cyt, relative to low-sodium controls (Fig. 6), under all levels of K+ supply. This effect was somewhat alleviated with increasing K+ provision, but, even at the highest [K+]ext, the pool size of K+ was reduced by nearly one-half. Again, this pattern of no reciprocal effect on cytosolic pool sizes of Na+ and K+ was confirmed in 2-week-old barley seedlings (not shown).
Fig. 6.
Cytosolic K+ activity in roots of barley seedlings grown under varying K+ provision, with or without 100 mM Na+. Error bars refer to ±SEM of 4–14 replicates. Asterisks denote significant differences within a given K+ treatment (P < 0.05).
Fig. 7.
Cytosolic Na+ activity in roots of barley seedlings grown under 100 mM Na+ and varying K+ provision. Error bars refer to ±SEM of four to seven replicates. No significant differences between treatments were observed.
Other workers, using techniques of X-ray microanalysis, radiotracer analysis, subcellular fractionation, and ion-selective microelectrodes, have reported very similar values for cytosolic activities of sodium (Harvey and Flowers, 1978; Yeo, 1981; Mills et al., 1985; Amtmann and Gradmann, 1994; Flowers and Hajibagheri, 2001; James et al., 2006) and potassium (Pitman and Saddler, 1967; Vorobiev, 1967; Macklon, 1975; Memon et al., 1985; Beilby and Blatt, 1986; Maathuis and Sanders, 1993; Walker et al., 1996). There have also been reports of suppressed cytosolic K+ pool by Na+ (Jeschke and Stelter, 1976; Harvey et al., 1981; Mills et al., 1985; Hajibagheri et al., 1987, 1988, 1989; Flowers and Hajibagheri, 2001; Carden et al., 2003; Chen et al., 2007a). A similar suppression of aK+,cyt has been observed to result from high ammonium (NH4+) provision in the same model system (Kronzucker et al., 2003), but, unlike the effect of Na+, this effect was only seen in the high-affinity K+ transport range (0.1 mM [K+]ext). By contrast, suppression of aK+,cyt by high Na+ was not observed at this level of K+ supply in previous work (Kronzucker et al., 2006), suggesting that K+ homeostasis is more resilient to salt stress in the high-affinity range.
The variability of the cytosolic K+ pool in response to both salinity and external K+ supply, and, by contrast, the uniformity of the Na+ pool with changes in external K+, together result in substantial (greater than 4-fold) changes in the ratio of the two cytosolic pools (Fig. 8). This K+:Na+ ratio peaked at 40 mM [K+]ext, and was minimized at 5 mM [K+]ext, but did not correlate with the growth of the experimental plants (Fig. 9A; also seen in 2-week-old plants, Fig. 9B). As with previous work (Kronzucker et al., 2006), this lack of correlation calls into question the view that the cytosolic K+:Na+ ratio is a critical determinant of plant growth in response to salinity stress (see Introduction). On the other hand, the ratio of K+ to Na+ on the coarser, tissue level, is rather uniform among salinity-treated plants (Fig. 8), as is the growth of plants under these conditions, indicating that this measure is indeed more of a more accurate predictor of plant performance under salt stress (see Introduction). This conclusion is supported by previously observed correlations between salinity tolerance and the tissue K+:Na+ ratio in other cultivars of barley (Flowers and Hajibagheri, 2001; Chen et al., 2007b). Interestingly, however, a recent study by Genc et al. (2007) showed that even this measure did not correlate with growth in 21 cultivars of bread wheat. The authors explained this as possibly reflecting alternative strategies of salt tolerance in different cereal species.
Fig. 8.
Cytosolic and tissue K+:Na+ ratios in roots of intact barley seedlings, grown at 100 mM Na+ and varying K+ provision.
In summary, neither Na+ fluxes nor cytosolic Na+ pools responded to K+ over a wide range of K+ supply [400-fold, when results from a previous work (Kronzucker et al., 2006) are included], and elevated K+ provision was unable to rescue barley plants from the primary toxic entry of Na+, contrary to the rescue from NH4+ toxicity that is effected by increasing external provision of K+ (Britto and Kronzucker, 2002; Kronzucker et al., 2003; Szczerba et al., 2006). The non-reciprocal nature of potassium–sodium interactions in barley provides insight into the mediation of primary Na+ entry into plant roots under toxicity-inducing conditions, an unresolved issue under much current debate (Schachtman and Liu, 1999; Golldack et al., 2003; Tester and Davenport, 2003; Qi and Spalding, 2004; Fuchs et al., 2005; Kader and Lindberg, 2005; Flowers, 2006; Davenport et al., 2007; Obata et al., 2007; Takahashi et al., 2007; Wang et al., 2007).
The present results suggest that several ion transporters favoured as candidates in the recent literature should likely be discounted, at least in barley. These include, on the one hand, K+ channels, such as less selective members of the AKT family of transporters, and, on the other, NSCCs and the low-affinity cation transporter LCT, which have been shown to allow the passage of a variety of cations under certain conditions (Amtmann and Sanders, 1999; Maathuis and Amtmann, 1999; Uozumi et al., 2000; Amtmann et al., 2001; Rus et al., 2001; Kader and Lindberg, 2005; Kader et al., 2006; Wang et al., 2007). Were members of these families of transporters, or of high-affinity K+ transporters (Takahashi et al., 2007), critically involved in toxic Na+ influx, increasing external K+ concentrations over an extensive range as employed in the present study would be expected to strongly reduce Na+ influx by virtue of ion competition effects. While background levels of Ca2+ were high in the present study (5 mM), and it is recognized that this may have minimized to some extent Na+ influx contribution from NSCCs and LCT transporters that are known to be Ca2+-sensitive (see Rains and Epstein, 1967; Schachtman and Liu, 1999; Amtmann et al., 2001; Essah et al., 2003; Wang et al., 2006; Davenport et al., 2007; Wang et al., 2007), it should be pointed out that Ca2+ levels in soils are also typically in excess of 1 mM (Reisenauer, 1966; Hawkesford and Miller, 2004). Thus, if a major contribution from NSCCs or LCT cannot be realized or rationalized under such conditions, the contribution from these transporters may also not be particularly relevant in the field (Schachtman and Liu, 1999; for a similar conclusion, also see Wang et al., 2007). Two recent studies have also suggested that K+-specific Shaker-type channels are unlikely candidates for Na+ influx; in one case, a knockout mutation of AKT1 in Arabidopsis thaliana resulted in reduced growth under Na+ stress, showing that toxic entry of Na+ can still proceed in the absence of AKT1 (Qi and Spalding, 2004). In the other case, overexpression of KAT1 in yeast cells resulted in lowered Na+ accumulation (Obata et al., 2007). Another group of transporters whose role in primary Na+ influx has been rigorously discussed is the HKT family. It has been proposed that the most well-studied member of this group, HKT1;1 (Rubio et al., 1995) is a major determinant of sodium influx in Arabidopsis (Rus et al., 2001), but other reports suggest that its function is in long-distance recirculation of Na+ (Berthomieu et al., 2003; Davenport et al., 2007). The strong suppression of HKT2-mediated Na+ influx by even low amounts of external K+ in grasses (Laurie et al., 2002; Horie et al., 2007) suggests that this transporter is not likely to play a significant role in Na+ uptake under normal soil conditions or in the present study.
However, other transporter types may emerge as mediators of toxic Na+ entry into the plant (Horie et al., 2001; Golldack et al., 2002, 2003; Garciadeblás et al., 2003; Haro et al., 2005; Ren et al., 2005; Kader et al., 2006). In particular, the involvement of sodium-specific influx transporters remains a possibility, even though such systems have not yet been identified through Arabidopsis genomic analysis (see Hua et al., 2003). Another possibility may involve the coupled transport of Na+ with ions such as chloride. A transporter of this kind has recently been identified in Arabidopsis (Colmenero-Flores et al., 2007).
Clearly, however, a definitive answer has not been forthcoming, and the search for the primary Na+ influx transporter mediating toxic plasma-membrane influx continues (Flowers, 2006). The present study indicates that, in barley, this transporter (or transport system) appears to be K+-independent, suggesting that attempts to improve salt tolerance in this species, via increased selectivity of K+ uptake pathways, are unlikely to be effective.
Acknowledgments
We thank M Butler at the McMaster Nuclear Research Reactor for providing 24Na+ and 42K+ tracers, SA Ali, A Bettio, P Malagoli, and A Versterberg for assistance in experiments. The work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC), the International Plant Nutrition Institute [formerly the Potash & Phosphate Institute (PPI)], and the Canada Research Chair (CRC) program.
References
- Amtmann A, Fischer M, Marsh EL, Stefanovic A, Sanders D, Schachtman DP. The wheat cDNA LCT1 generates hypersensitivity to sodium in a salt-sensitive yeast strain. Plant Physiology. 2001;126:1061–1071. doi: 10.1104/pp.126.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amtmann A, Gradmann D. Na+ transport in Acetabularia bypasses conductance of plasmalemma. Journal of Membrane Biology. 1994;139:117–125. doi: 10.1007/BF00232430. [DOI] [PubMed] [Google Scholar]
- Amtmann A, Sanders D. Mechanisms of Na+ uptake by plant cells. Advances in Botanical Research. 1999;29:75–112. [Google Scholar]
- Beilby MJ, Blatt MR. Simultaneous measurements of cytoplasmic potassium concentration and the plasma membrane electrical parameters in single membrane samples of Chara corallina. Plant Physiology. 1986;82:417–422. doi: 10.1104/pp.82.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthomieu P, Conejero G, Nublat A, et al. Functional analysis of AtHKT1 in Arabidopsis shows that Na+ recirculation by the phloem is crucial for salt tolerance. EMBO Journal. 2003;22:2004–2014. doi: 10.1093/emboj/cdg207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britto DT, Kronzucker HJ. NH4+ toxicity in higher plants: a critical review. Journal of Plant Physiology. 2002;159:567–584. [Google Scholar]
- Britto DT, Siddiqi MY, Glass ADM, Kronzucker HJ. Futile transmembrane NH4+ cycling: a cellular hypothesis to explain ammonium toxicity in plants. Proceedings of the National Academy of Sciences, USA. 2001;98:4255–4258. doi: 10.1073/pnas.061034698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britto DT, Szczerba MW, Kronzucker HJ. A new, non-perturbing, sampling procedure in tracer exchange measurements. Journal of Experimental Botany. 2006;57:1309–1314. doi: 10.1093/jxb/erj105. [DOI] [PubMed] [Google Scholar]
- Cakmak I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. Journal of Plant Nutrition and Soil Science. 2005;168:521–530. [Google Scholar]
- Carden DE, Walker DJ, Flowers TJ, Miller AJ. Single-cell measurements of the contributions of cytosolic Na+ and K+ to salt tolerance. Plant Physiology. 2003;131:676–683. doi: 10.1104/pp.011445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman JM. Pump-leak sodium fluxes in low salt corn roots. Journal of Membrane Biology. 1982;70:157–164. [Google Scholar]
- Chen Z, Newman I, Zhou M, Mendham N, Zhang G, Shabala S. Screening plants for salt tolerance by measuring K+ flux: a case study for barley. Plant, Cell and Environment. 2005;28:1230–1246. [Google Scholar]
- Chen Z, Pottosin II, Cuin TA, et al. Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley. Plant Physiology. 2007a;145:1714–1725. doi: 10.1104/pp.107.110262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Zhou M, Newman IA, Mendham NJ, Zhang G, Shabala S. Potassium and sodium relations in salinised barley tissues as a basis of differential salt tolerance. Functional Plant Biology. 2007b;34:150–162. doi: 10.1071/FP06237. [DOI] [PubMed] [Google Scholar]
- Colmenero-Flores JM, Martinez G, Gamba G, Vazquez N, Iglesias DJ, Javier Brumos, Talon M. Identification and functional characterization of cation-chloride cotransporters in plants. The Plant Journal. 2007;50:278–292. doi: 10.1111/j.1365-313X.2007.03048.x. [DOI] [PubMed] [Google Scholar]
- Cuin TA, Shabala S. Amino acids regulate salinity-induced potassium efflux in barley root epidermis. Planta. 2007;225:753–761. doi: 10.1007/s00425-006-0386-x. [DOI] [PubMed] [Google Scholar]
- Davenport RJ, Munoz-Mayor A, Jha D, Essah PA, Rus A, Tester M. The Na+ transporter AtHKT1;1 controls retrieval of Na+ from the xylem in Arabidopsis. Plant, Cell and Environment. 2007;30:497–507. doi: 10.1111/j.1365-3040.2007.01637.x. [DOI] [PubMed] [Google Scholar]
- Deal KR, Goyal S, Dvorak J. Arm location of Lophopyrum elongatum genes affecting K+/Na+ selectivity under salt stress. Euphytica. 1999;108:193–198. [Google Scholar]
- Demidchik V, Davenport RJ, Tester M. Nonselective cation channels in plants. Annual Review of Plant Biology. 2002;53:67–107. doi: 10.1146/annurev.arplant.53.091901.161540. [DOI] [PubMed] [Google Scholar]
- Epstein E, Norlyn JD, Rush DW, Kingsbury RW, Kelley DB, Cunningham GA, Wrona AF. Saline culture of crops: a genetic approach. Science. 1980;210:399–404. doi: 10.1126/science.210.4468.399. [DOI] [PubMed] [Google Scholar]
- Epstein E, Rains DW, Elzam OE. Resolution of dual mechanisms of potassium absorption by barley roots. Proceedings of the National Academy of Sciences, USA. 1963;49:684–692. doi: 10.1073/pnas.49.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essah PA, Davenport R, Tester M. Sodium influx and accumulation in Arabidopsis. Plant Physiology. 2003;133:307–318. doi: 10.1104/pp.103.022178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers T. Plants and salinity – preface. Journal of Experimental Botany. 2006;57 (Special Issue: Plants and Salinity), iv. [Google Scholar]
- Flowers TJ, Hajibagheri MA. Salinity tolerance in Hordeum vulgare: ion concentrations in root cells of cultivars differing in salt tolerance. Plant and Soil. 2001;231:1–9. [Google Scholar]
- Flowers TJ, Läuchli A. Sodium versus potassium: substitution and compartmentation. In: Läuchli A, Bieleski RL, editors. Encyclopedia of plant physiology. Vol. 15B. Berlin: Springer-Verlag; 1983. pp. 651–680. New Series. [Google Scholar]
- Fu HH, Luan S. AtKUP1: A dual-affinity K+ transporter from Arabidopsis. The Plant Cell. 1998;10:63–73. doi: 10.1105/tpc.10.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs I, Stolzle S, Ivashikina N, Hedrich R. Rice K+ uptake channel OsAKT1 is sensitive to salt stress. Planta. 2005;221:212–221. doi: 10.1007/s00425-004-1437-9. [DOI] [PubMed] [Google Scholar]
- Garciadeblás B, Senn ME, Bañuelos MA, Rodríguez-Navarro A. Sodium transport and HKT transporters: the rice model. The Plant Journal. 2003;34:788–801. doi: 10.1046/j.1365-313x.2003.01764.x. [DOI] [PubMed] [Google Scholar]
- Gaxiola R, De Larrinoa IF, Villalba JM, Serrano R. A novel and conserved salt-induced protein is an important determinant of salt tolerance in yeast. EMBO Journal. 1992;11:3157–3164. doi: 10.1002/j.1460-2075.1992.tb05392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genc Y, McDonald GK, Tester M. Reassessment of tissue Na+ concentration as a criterion for salinity tolerance in bread wheat. Plant, Cell and Environment. 2007;30:1486–1498. doi: 10.1111/j.1365-3040.2007.01726.x. [DOI] [PubMed] [Google Scholar]
- Golldack D, Quigley F, Michalowski CB, Kamasani UR, Bohnert HJ. Salinity stress-tolerant and -sensitive rice (Oryza sativa L.) regulate AKT1-type potassium channel transcripts differently. Plant Molecular Biology. 2003;51:71–81. doi: 10.1023/a:1020763218045. [DOI] [PubMed] [Google Scholar]
- Golldack D, Su H, Quigley F, Kamasani UR, Munoz-Garay C, Balderas E, Popova OV, Bennett J, Bohnert HJ, Pantoja O. Characterization of a HKT-type transporter in rice as a general alkali cation transporter. The Plant Journal. 2002;31:529–542. doi: 10.1046/j.1365-313x.2002.01374.x. [DOI] [PubMed] [Google Scholar]
- Hajibagheri MA, Flowers TJ, Collins JC, Yeo AR. A comparison of the methods of X-ray-microanalysis, compartmental analysis and longitudinal ion profiles to estimate cytoplasmic ion concentrations in two maize varieties. Journal of Experimental Botany. 1988;39:279–290. [Google Scholar]
- Hajibagheri MA, Harvey DMR, Flowers TJ. Quantitative ion distribution within root-cells of salt-sensitive and salt-tolerant maize varieties. New Phytologist. 1987;105:367–379. doi: 10.1111/j.1469-8137.1987.tb00874.x. [DOI] [PubMed] [Google Scholar]
- Hajibagheri MA, Yeo AR, Flowers TJ, Collins JC. Salinity resistance in Zea mays – fluxes of potassium, sodium and chloride, cytoplasmic concentrations and microsomal membrane-lipids. Plant, Cell and Environment. 1989;12:753–757. [Google Scholar]
- Haro R, Bañuelos MA, Senn ME, Barrero-Gil J, Rodríguez-Navarro A. HKT1 mediates sodium uniport in roots: pitfalls in the expression of HKT1 in yeast. Plant Physiology. 2005;139:1495–1506. doi: 10.1104/pp.105.067553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey DRM, Flowers TJ. Determination of the sodium, potassium and chloride ion concentrations in the chloroplasts of the halophyte Suaeda maritima by non-aqueous cell fractionation. Protoplasma. 1978;97:337–349. [Google Scholar]
- Harvey DMR, Hall JL, Flowers TJ, Kent B. Quantitative ion localization within Suaeda maritima leaf mesophyll cells. Planta. 1981;151:555–560. doi: 10.1007/BF00387435. [DOI] [PubMed] [Google Scholar]
- Hawkesford MJ, Miller AJ. Ion-coupled transport of inorganic solutes. In: Blatt MR, editor. Membrane transport in plants. Annual Plant Reviews. Vol. 15. Oxford: Blackwell Publishing; 2004. pp. 105–134. [Google Scholar]
- Horie T, Costa A, Kim TH, Han MJ, Horie R, Leung H-Y, Miyai A, Hirochika H, An G, Schroedr JI. Rice OsHKT2;1 transporter mediates large Na+ influx component into K+-starved roots for growth. EMBO Journal. 2007;26:3003–3014. doi: 10.1038/sj.emboj.7601732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie T, Yoshida K, Nakayama H, Yamada K, Oiki S, Shinmyo A. Two types of HKT transporters with different properties of Na+ and K+ transport in Oryza sativa. The Plant Journal. 2001;27:129–138. doi: 10.1046/j.1365-313x.2001.01077.x. [DOI] [PubMed] [Google Scholar]
- Hua BG, Mercier RW, Leng Q, Berkowitz GA. Plants do it differently: a new basis for potassium/sodium selectivity in the pore of an ion channel. Plant Physiology. 2003;132:1353–1361. doi: 10.1104/pp.103.020560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James RA, Munns R, Von Caemmerer S, Trejo C, Miller C, Condon T. Photosynthetic capacity is related to the cellular and subcellular partitioning of Na+, K+ and Cl– in salt-affected barley and durum wheat. Plant, Cell and Environment. 2006;26:2185–2197. doi: 10.1111/j.1365-3040.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- Jander G, Blasius E. Lehrbuch der Analytischen und Präparativen Anorganischen Chemie. 12th edn. Stuttgart: S. Hirzel Publishing House; 1988. pp. 54–55. [Google Scholar]
- Jeschke WD, Stelter W. Measurement of longitudinal ion profiles in single roots of Hordeum and Atriplex by use of flameless atomic absorption spectroscopy. Planta. 1976;128:1076–1080. doi: 10.1007/BF00390311. [DOI] [PubMed] [Google Scholar]
- Kader MA, Lindberg S. Uptake of sodium in protoplasts of salt-sensitive and salt-tolerant cultivars of rice, Oryza sativa L. determined by the fluorescent dye SBFI. Journal of Experimental Botany. 2005;56:3149–3158. doi: 10.1093/jxb/eri312. [DOI] [PubMed] [Google Scholar]
- Kader MA, Seidel T, Golldack D, Lindberg S. Expressions of OsHKT1, OsHKT2, and OsVHA are differentially regulated under NaCl stress in salt-sensitive and salt-tolerant rice (Oryza sativa L.) cultivars. Journal of Experimental Botany. 2006;57:4257–4268. doi: 10.1093/jxb/erl199. [DOI] [PubMed] [Google Scholar]
- Kochian LV, Lucas WJ. Potassium transport in corn roots. I. Resolution of kinetics into a saturable and linear component. Plant Physiology. 1982;70:1723–1731. doi: 10.1104/pp.70.6.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV, Xin-zhi J, Lucas W. Potassium transport in corn roots. IV. Characterization of the linear component. Plant Physiology. 1985;79:771–776. doi: 10.1104/pp.79.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker HJ, Glass ADM, Siddiqi MY. Inhibition of nitrate uptake by ammonium in barley: analysis of component fluxes. Plant Physiology. 1999;120:283–292. doi: 10.1104/pp.120.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker HJ, Szczerba MW, Britto DT. Cytosolic potassium homeostasis revisited: 42K-tracer analysis in Hordeum vulgare L. reveals set-point variations in [K+] Planta. 2003;217:540–546. doi: 10.1007/s00425-003-1032-5. [DOI] [PubMed] [Google Scholar]
- Kronzucker HJ, Szczerba MW, Moazami-Goudarzi M, Britto DT. The cytosolic Na+:K+ ratio does not explain salinity-induced growth impairment in barley: a dual-tracer study using 42K+ and 24Na+ Plant, Cell and Environment. 2006;29:2228–2237. doi: 10.1111/j.1365-3040.2006.01597.x. [DOI] [PubMed] [Google Scholar]
- Laurie S, Feeney KA, Maathuis FJM, Heard PJ, Brown SJ, Leigh RA. A role for HKT1 in sodium uptake by wheat roots. The Plant Journal. 2002;32:139–149. doi: 10.1046/j.1365-313x.2002.01410.x. [DOI] [PubMed] [Google Scholar]
- Maathuis FJM, Amtmann A. K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Annals of Botany. 1999;84:123–133. [Google Scholar]
- Maathuis FJM, Sanders D. Energization of potassium uptake in Arabidopsis thaliana. Planta. 1993;191:302–307. [Google Scholar]
- Macklon AES. Cortical cell fluxes and transport to stele in excised root segments of Allium cepa L. 1. Potassium, sodium and chloride. Planta. 1975;122:109–130. doi: 10.1007/BF00388652. [DOI] [PubMed] [Google Scholar]
- Martínez-Cordero A, Martínez V, Rubio F. High-affinity K+ uptake in pepper plants. Journal of Experimental Botany. 2005;56:1553–1562. doi: 10.1093/jxb/eri150. [DOI] [PubMed] [Google Scholar]
- Memon AR, Saccomani M, Glass ADM. Efficiency of potassium utilization by barley Hordeum vulgare varieties the role of subcellular compartmentation. Journal of Experimental Botany. 1985;36:1860–1876. [Google Scholar]
- Mills D, Robinson K, Hodges TK. Sodium and potassium fluxes and compartmentation in roots of Atriplex and oat. Plant Physiology. 1985;78:500–509. doi: 10.1104/pp.78.3.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns RA. Genes and salt tolerance: bringing them together. New Phytologist. 2005;167:645–663. doi: 10.1111/j.1469-8137.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- Obata T, Kitamoto HK, Nakamura A, Fukuda A, Tanaka Y. Rice shaker potassium channel OsKAT1 confers tolerance to salinity stress on yeast and rice cells. Plant Physiology. 2007;144:1978–1985. doi: 10.1104/pp.107.101154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y-H, Zhu Y-F, Mao Y-Q, Wang S-M, Su W-A, Tang Z-C. Alkali grass resists salt stress through high [K+] and an endodermis barrier to Na+ Journal of Experimental Botany. 2004;55:939–949. doi: 10.1093/jxb/erh071. [DOI] [PubMed] [Google Scholar]
- Pitman MG, Saddler HDW. Active sodium and potassium transport in cells of barley roots. Proceedings of the National Academy of Sciences, USA. 1967;57:44–49. doi: 10.1073/pnas.57.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, Spalding EP. Protection of plasma membrane K+ transport by the salt overly sensitive Na+-H+ antiporter curing salinity stress. Plant Physiology. 2004;136:2548–2555. doi: 10.1104/pp.104.049213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rains DW, Epstein E. Sodium absorption by barley roots: its mediation by mechanism 2 of alkali cation transport. Plant Physiology. 1967;42:319–323. doi: 10.1104/pp.42.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisenauer HM. Mineral nutrients in soil solution. Federation of American Societies for Experimental Biology. In: Altman PL, Ditter DS, editors. Environmental biology. Bethesda, MD: 1966. pp. 507–508. [Google Scholar]
- Ren ZH, Gao JP, Li LG, Cai XL, Huang W, Chao DY, Zhu MZ, Wang ZY, Luan S, Lin HX. A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nature Genetics. 2005;37:1141–1146. doi: 10.1038/ng1643. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Navarro A, Rubio F. High-affinity potassium and sodium transport systems in plants. Journal of Experimental Botany. 2006;57:1149–1160. doi: 10.1093/jxb/erj068. [DOI] [PubMed] [Google Scholar]
- Rubio F, Flores P, Navarro JM, Martínez V. Effects of Ca2+, K+ and cGMP on Na+ uptake in pepper plants. Plant Science. 2003;165:1043–1049. [Google Scholar]
- Rubio F, Gassmann W, Schroeder JI. Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science. 1995;270:1660–1663. doi: 10.1126/science.270.5242.1660. [DOI] [PubMed] [Google Scholar]
- Rubio F, Santa-María GE, Rodríguez-Navarro A. Cloning of Arabidopsis and barley cDNAs encoding HAK potassium transporters in root and shoot cells. Physiologia Plantarum. 2000;109:34–43. [Google Scholar]
- Rus A, Yokoi S, Sharkhuu A, Reddy M, Lee BH, Matsumoto TK, Koiwa H, Zhu JK, Bressan RA, Hasegawa PM. AtHKT1 is a salt tolerance determinant that controls Na+ entry into plant roots. Proceedings of the National Academy of Sciences, USA. 2001;98:14150–14155. doi: 10.1073/pnas.241501798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa-María GE, Epstein E. Potassium/sodium selectivity in wheat and the amphiploid cross wheat×Lophopyrum elongatum. Plant Science. 2001;160:523–534. doi: 10.1016/s0168-9452(00)00419-2. [DOI] [PubMed] [Google Scholar]
- Santa-María GE, Rubio F, Dubcovsky J, Rodríguez-Navarro A. The HAK1 gene of barley is a member of a large gene family and encodes a high-affinity potassium transporter. The Plant Cell. 1997;9:2281–2289. doi: 10.1105/tpc.9.12.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman DP, Liu WH. Molecular pieces to the puzzle of the interaction between potassium and sodium uptake in plants. Trends in Plant Science. 1999;4:281–287. doi: 10.1016/s1360-1385(99)01428-4. [DOI] [PubMed] [Google Scholar]
- Szczerba MW, Britto DT, Kronzucker HJ. Rapid, futile K+ cycling and pool-size dynamics define low-affinity potassium transport in barley. Plant Physiology. 2006;141:1494–1507. doi: 10.1104/pp.106.082701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi R, Nishio T, Ichizen N, Takano T. Cloning and functional analysis of the K+ transporter, PhaHAK2, from salt-sensitive and salt-tolerant reed plants. Biotechnology Letters. 2007;29:501–506. doi: 10.1007/s10529-006-9246-9. [DOI] [PubMed] [Google Scholar]
- Tester M, Davenport R. Na+ tolerance and Na+ transport in higher plants. Annals of Botany. 2003;91:503–527. doi: 10.1093/aob/mcg058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uozumi N, Kim EJ, Rubio F, Yamaguchi T, Muto S, Tsuboi A, Bakker EP, Nakamura T, Schroeder JI. The Arabidopsis HKT1 gene homolog mediates inward Na+ currents in Xenopus laevis oocytes and Na+ uptake in Saccharomyces cerevisiae. Plant Physiology. 2000;122:1249–1260. doi: 10.1104/pp.122.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobiev LN. Potassium ion activity in cytoplasm and vacuole of cells of Chara and Griffithsia. Nature. 1967;216:1325–1327. doi: 10.1038/2161325a0. [DOI] [PubMed] [Google Scholar]
- Walker DJ, Leigh RA, Miller AJ. Potassium homeostasis in vacuolate plant cells. Proceedings of the National Academy of Sciences, USA. 1996;93:10510–10514. doi: 10.1073/pnas.93.19.10510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Davenport RJ, Volkov V, Amtmann A. Low unidirectional sodium influx into root cells restricts net sodium accumulation in Thellungiella halophila, a salt-tolerant relative of Arabidopsis thaliana. Journal of Experimental Botany. 2006;57:1161–1170. doi: 10.1093/jxb/erj116. [DOI] [PubMed] [Google Scholar]
- Wang S-M, Zhang J-L, Flowers TJ. Low-affinity Na+ uptake in the halophyte Suaeda maritima. Plant Physiology. 2007;145:559–571. doi: 10.1104/pp.107.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warne TR, Hickok LG, Kinraide TB, Vogelien DL. High salinity tolerance in the stl2 mutation of Ceratopteris richardii is associated with enhanced K+ influx and efflux. Plant, Cell and Environment. 1996;19:24–32. [Google Scholar]
- Watad AE, Reuveni M, Bressan RA, Hasegawa PM. Enhanced net K uptake capacity of NaCl-adapted cells. Plant Physiology. 1991;95:1265–1269. doi: 10.1104/pp.95.4.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo AR. Salt tolerance in the halophyte Suaeda maritima (L.) Dum.: intracellular compartmentation of ions. Journal of Experimental Botany. 1981;32:487–497. [Google Scholar]
- Zhu JK. Plant salt tolerance. Trends in Plant Science. 2001;6:66–71. doi: 10.1016/s1360-1385(00)01838-0. [DOI] [PubMed] [Google Scholar]
- Zhu JK, Hasegawa PM, Bressan RA. Molecular aspects of osmotic stress in plants. Critical Reviews in Plant Sciences. 1997;16:253–277. [Google Scholar]
- Zhu JK, Liu JP, Xiong LM. Genetic analysis of salt tolerance in Arabidopsis: Evidence for a critical role of potassium nutrition. The Plant Cell. 1998;10:1181–1191. doi: 10.1105/tpc.10.7.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]