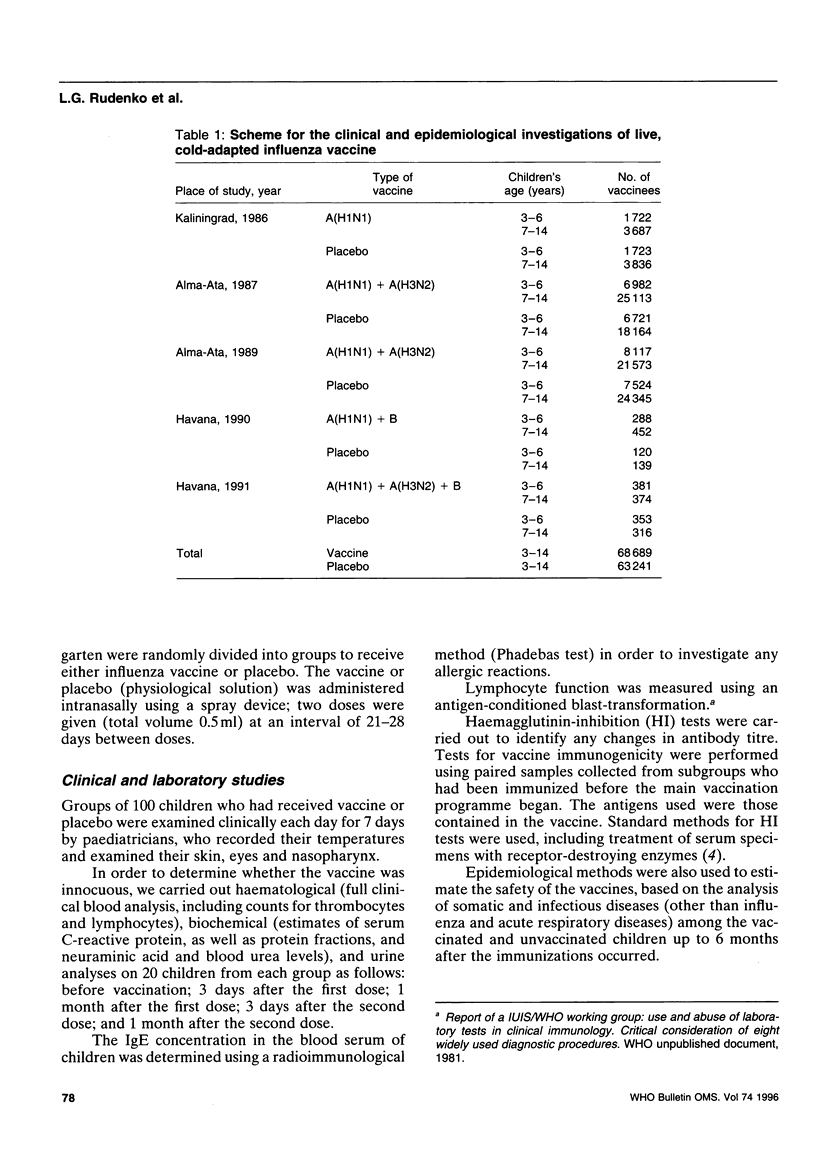
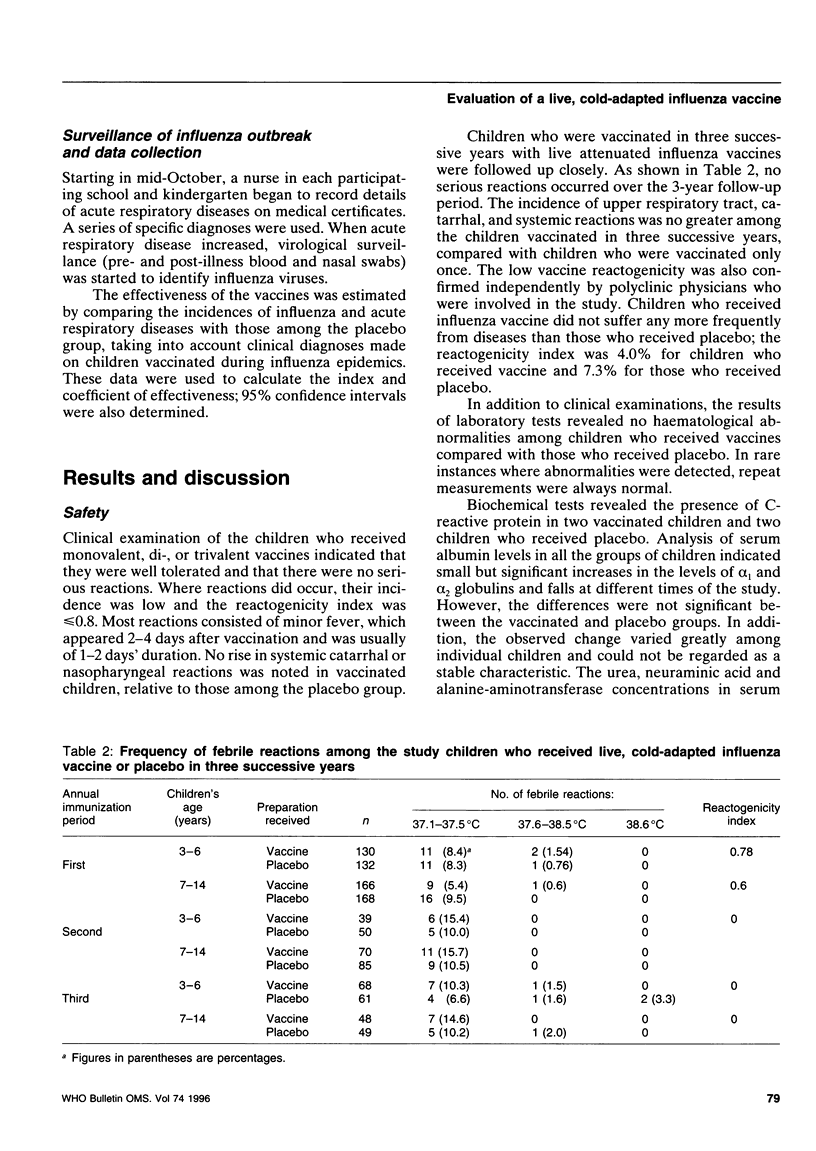
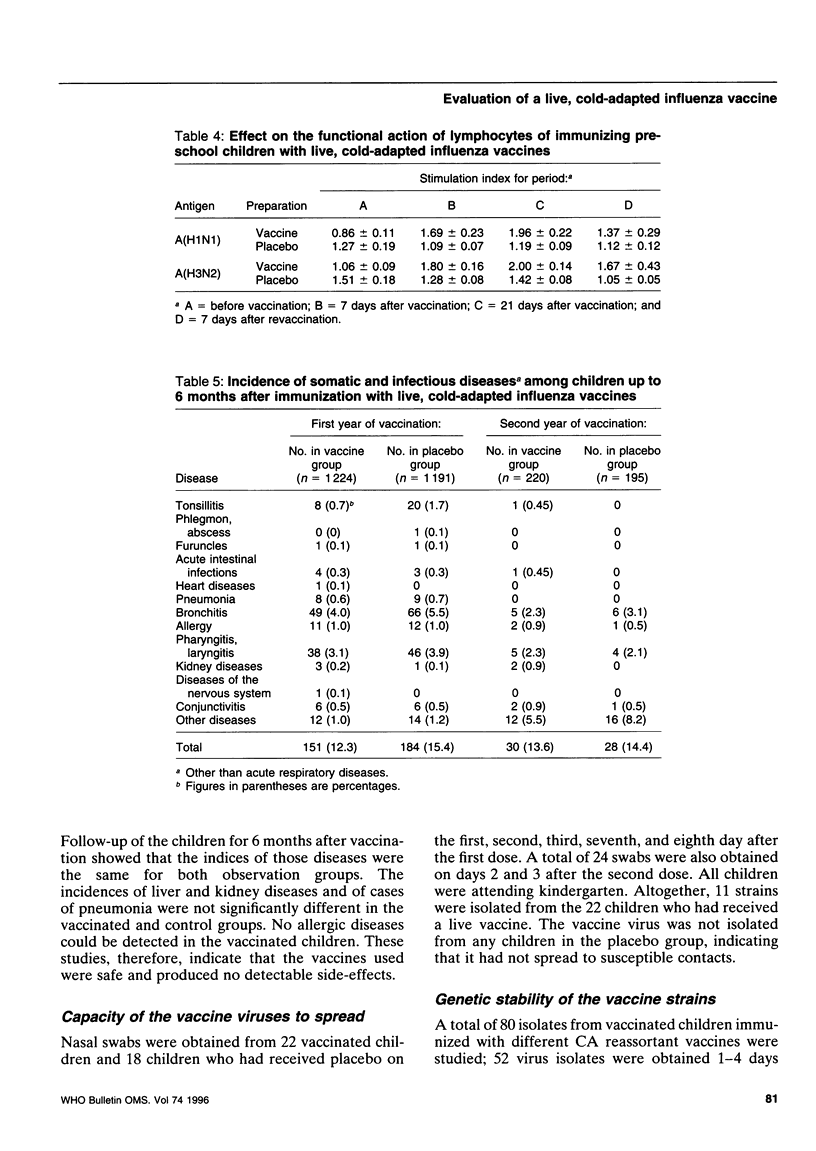
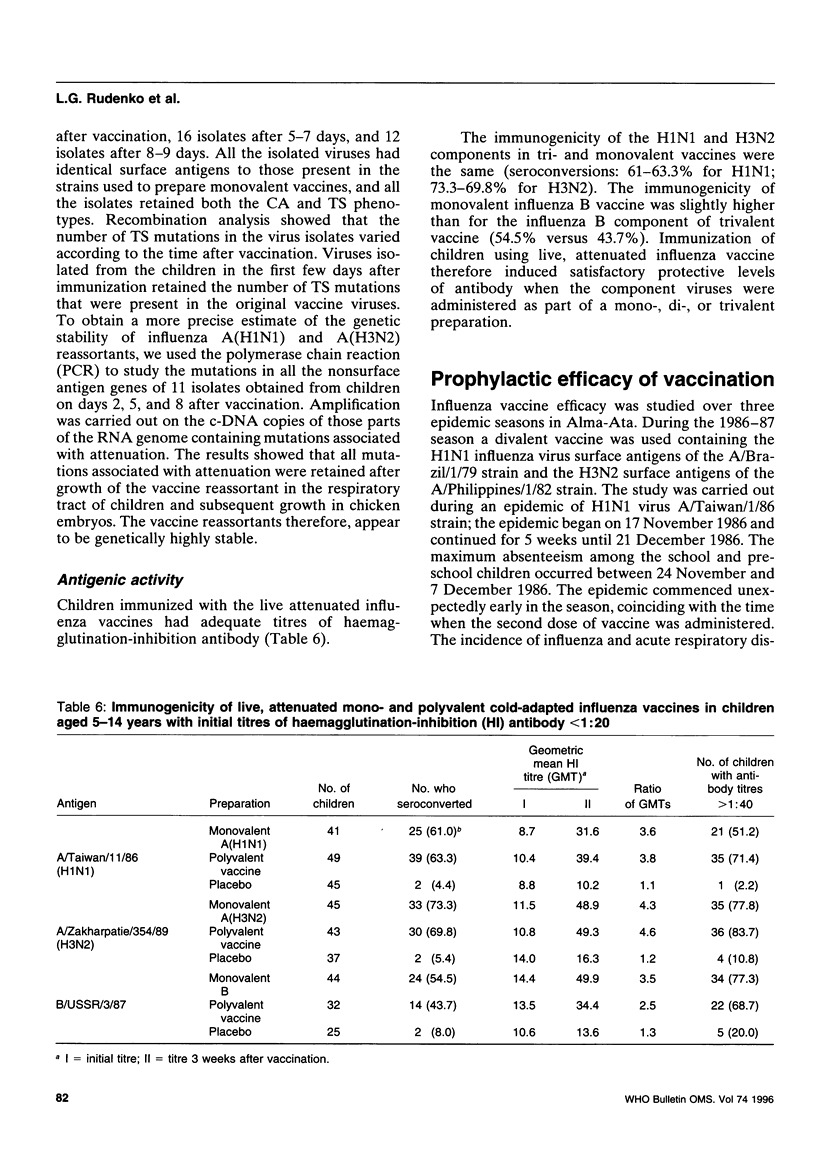
Abstract
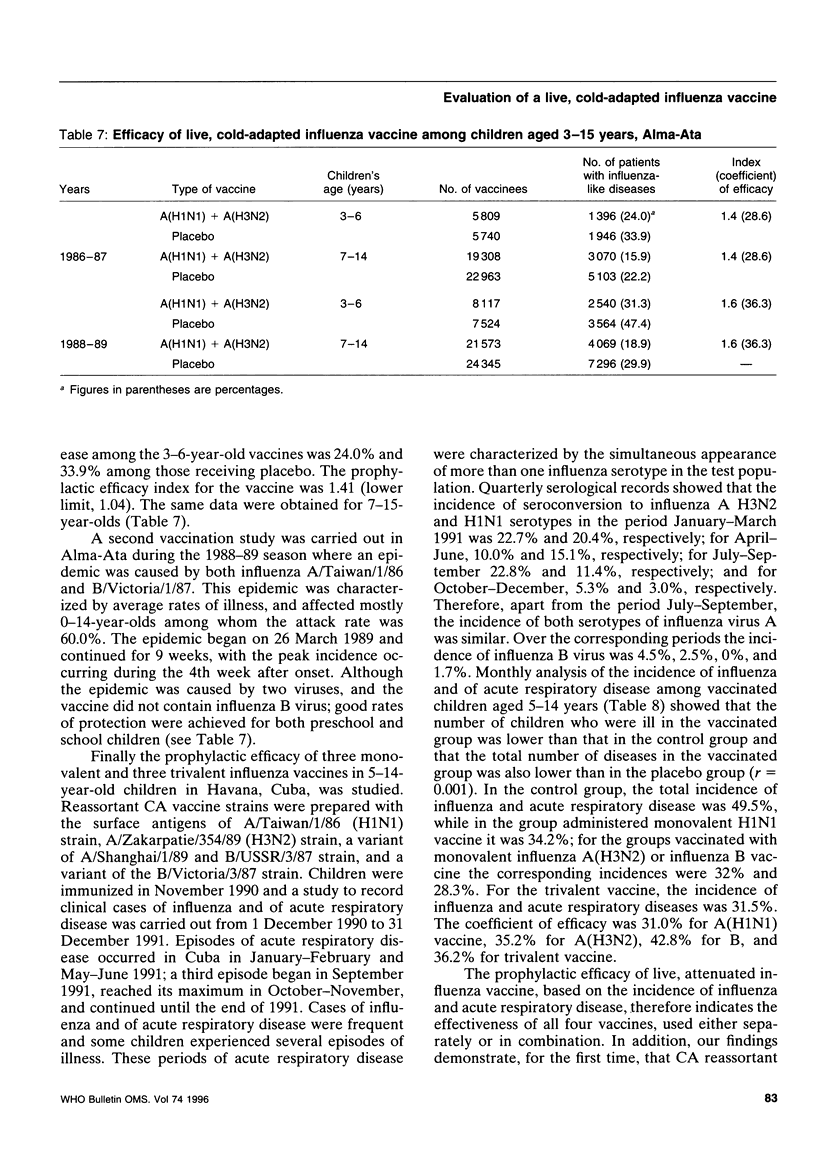
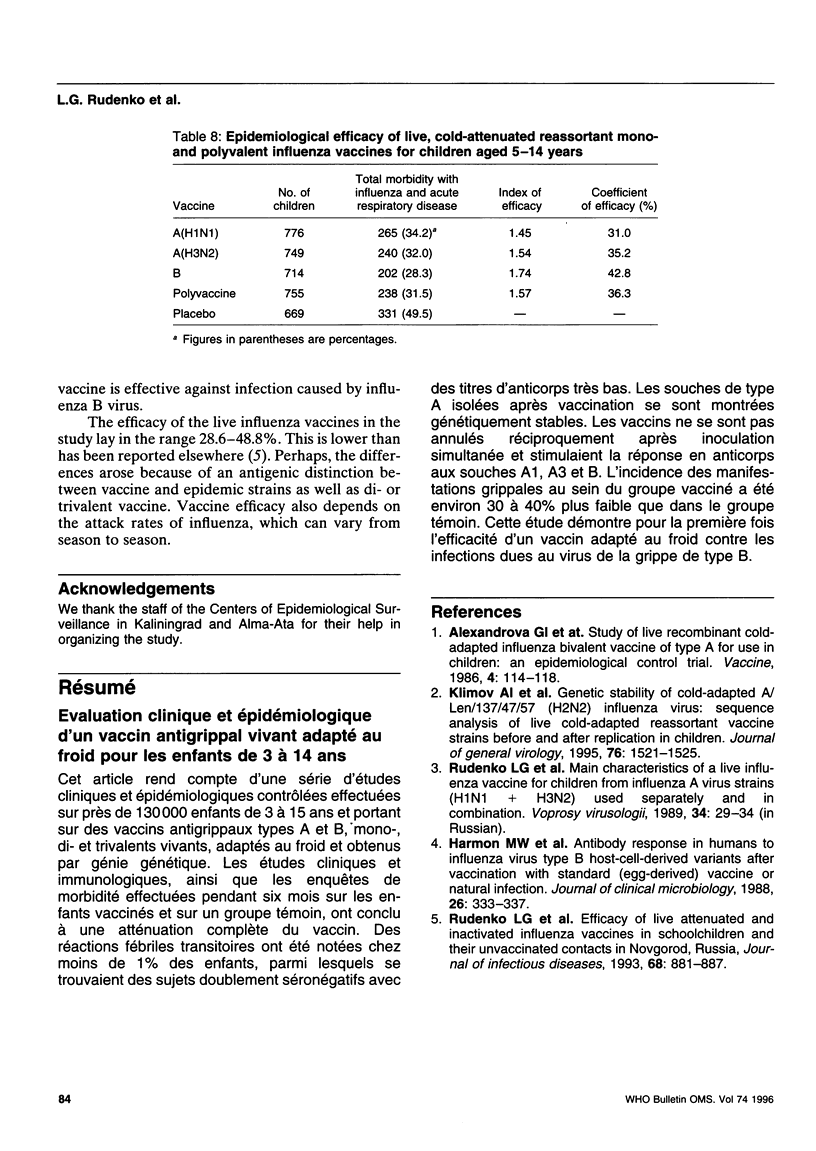
Reported is a study of live, cold-adapted (CA) reassortant mono-, di-, and trivalent influenza type A and B vaccines in a series of controlled clinical and epidemiological investigations involving nearly 130 000 children aged 3-15 years. The results of clinical, immunological, and morbidity investigations of the vaccinees and a control group over 6-months' follow-up indicated that the vaccines were completely attenuated by the children. Transient febrile reactions occurred in < 1% of the children after vaccination, including double seronegative individuals with low antibody titres. The type A reisolates examined were genetically stable. The reassortants did not suppress each other after simultaneous inoculation of children and stimulated antibody response to influenza virus strains A1, A3, and B. The incidence of influenza-like diseases was approximately 30-40% lower among the vaccinated group than among the control group. The study demonstrates, for the first time, the efficacy of CA vaccine against infections caused by influenza B virus.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandrova G. I., Budilovsky G. N., Koval T. A., Polezhaev F. I., Garmashova L. M., Ghendon YuZ, Romanova Y. R., Smorodintsev A. A. Study of live recombinant cold-adapted influenza bivalent vaccine of type A for use in children: an epidemiological control trial. Vaccine. 1986 Jun;4(2):114–118. doi: 10.1016/0264-410x(86)90049-6. [DOI] [PubMed] [Google Scholar]
- Harmon M. W., Rota P. A., Walls H. H., Kendal A. P. Antibody response in humans to influenza virus type B host-cell-derived variants after vaccination with standard (egg-derived) vaccine or natural infection. J Clin Microbiol. 1988 Feb;26(2):333–337. doi: 10.1128/jcm.26.2.333-337.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimov A. I., Egorov A. Y., Gushchina M. I., Medvedeva T. E., Gamble W. C., Rudenko L. G., Alexandrova G. I., Cox N. J. Genetic stability of cold-adapted A/Leningrad/134/47/57 (H2N2) influenza virus: sequence analysis of live cold-adapted reassortant vaccine strains before and after replication in children. J Gen Virol. 1995 Jun;76(Pt 6):1521–1525. doi: 10.1099/0022-1317-76-6-1521. [DOI] [PubMed] [Google Scholar]
- Rudenko L. G., Slepushkin A. N., Monto A. S., Kendal A. P., Grigorieva E. P., Burtseva E. P., Rekstin A. R., Beljaev A. L., Bragina V. E., Cox N. Efficacy of live attenuated and inactivated influenza vaccines in schoolchildren and their unvaccinated contacts in Novgorod, Russia. J Infect Dis. 1993 Oct;168(4):881–887. doi: 10.1093/infdis/168.4.881. [DOI] [PubMed] [Google Scholar]


