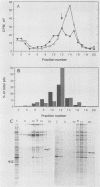
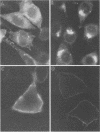
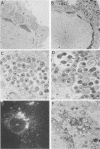
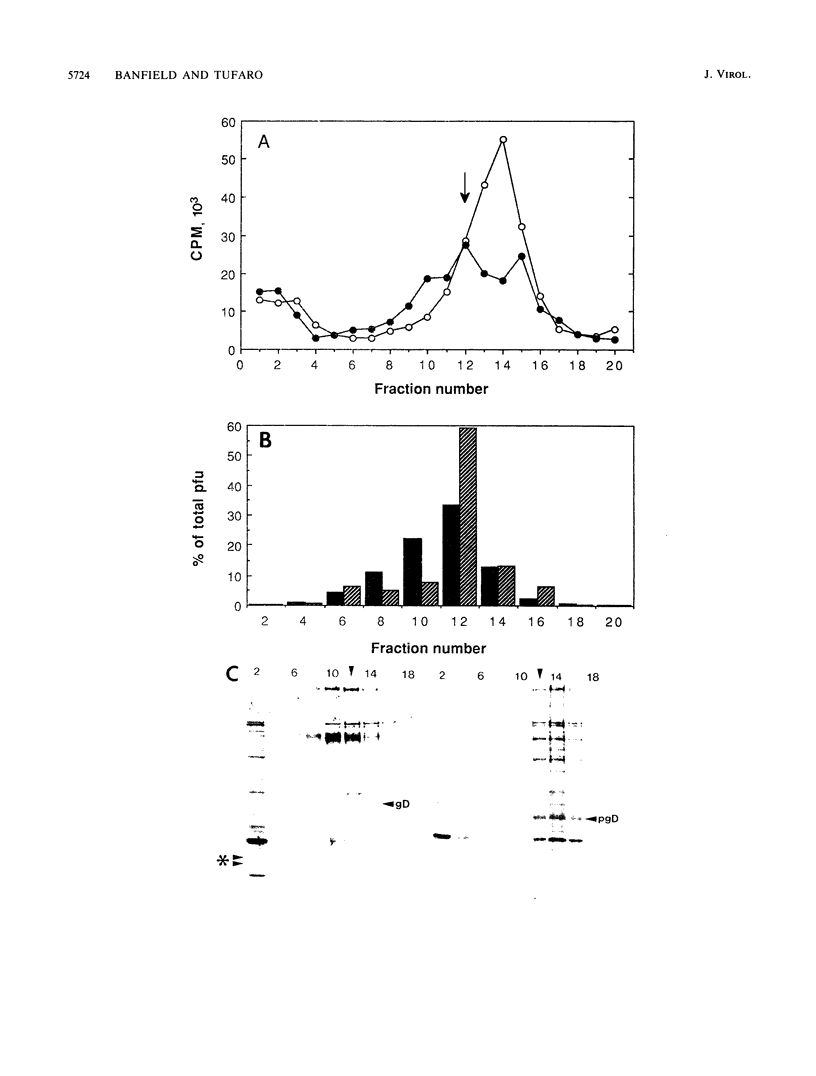
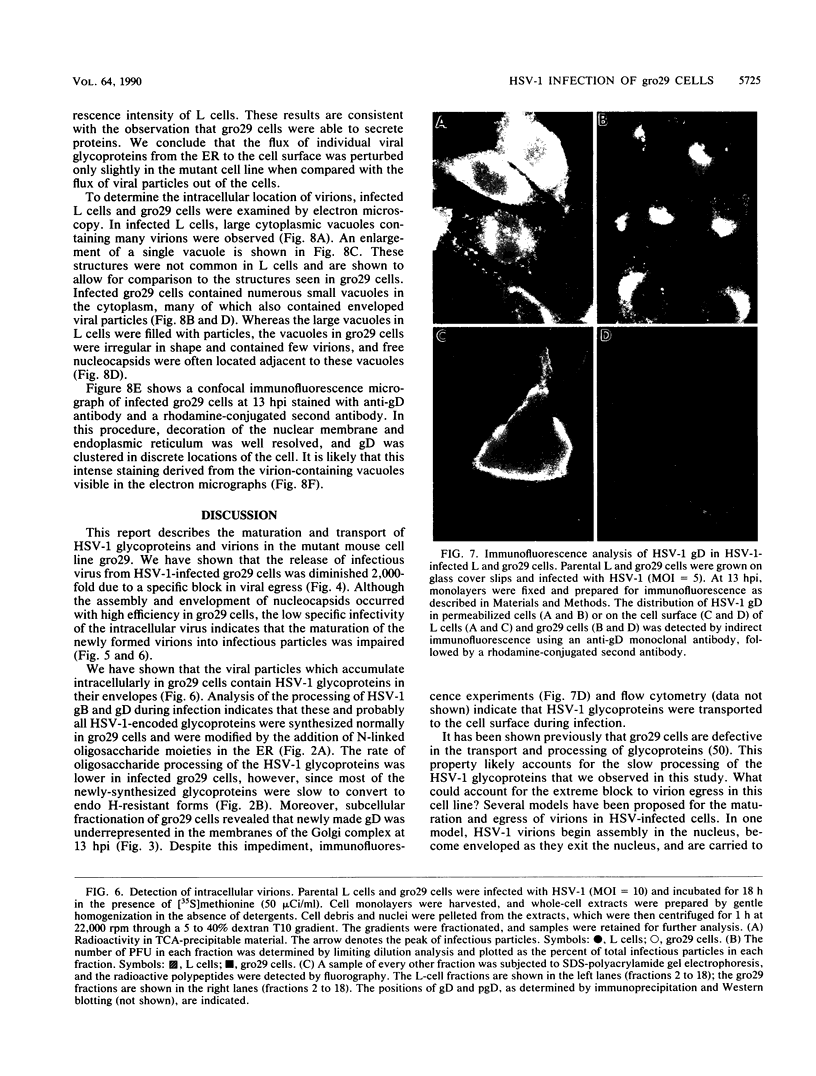
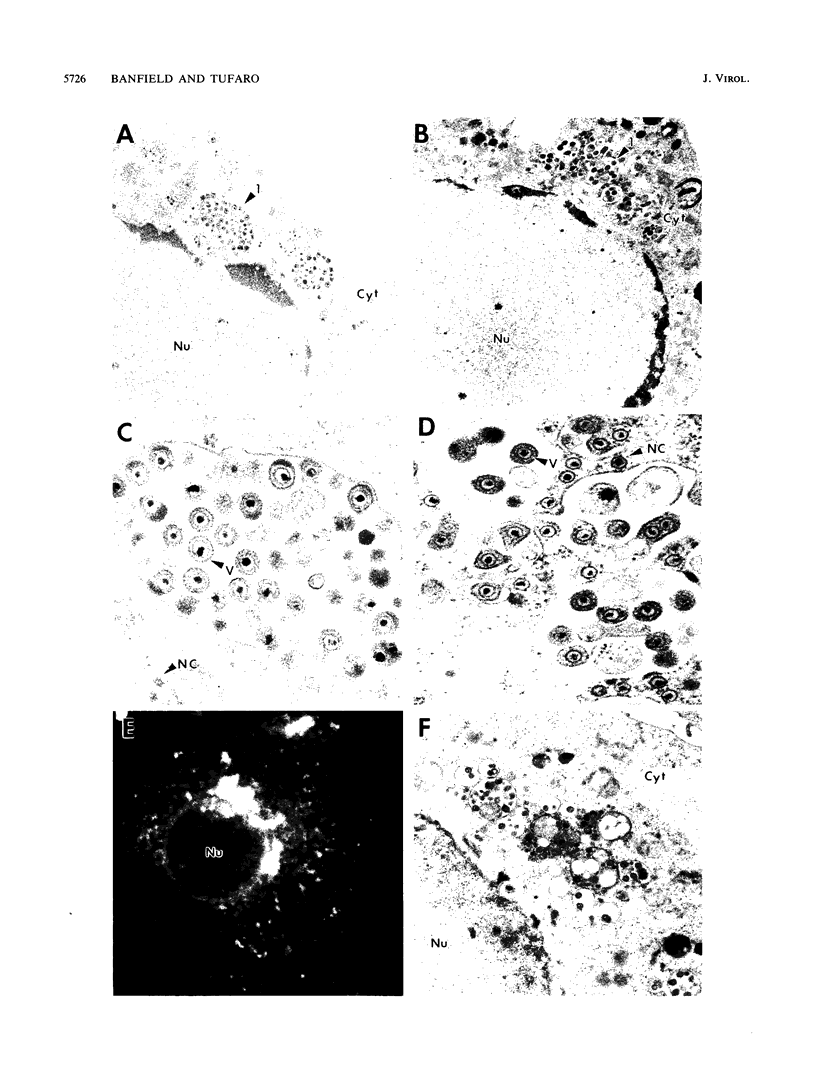
Abstract
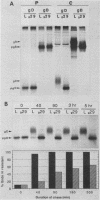
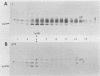
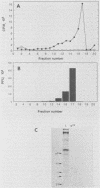
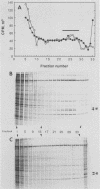
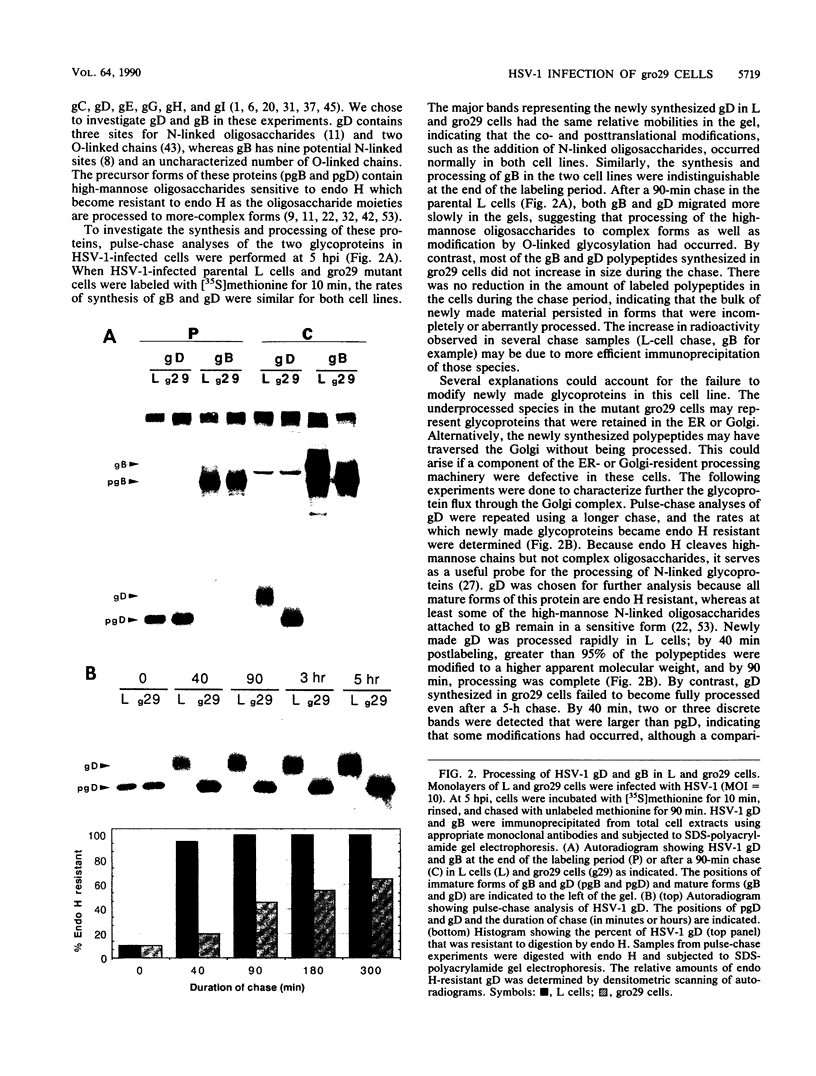
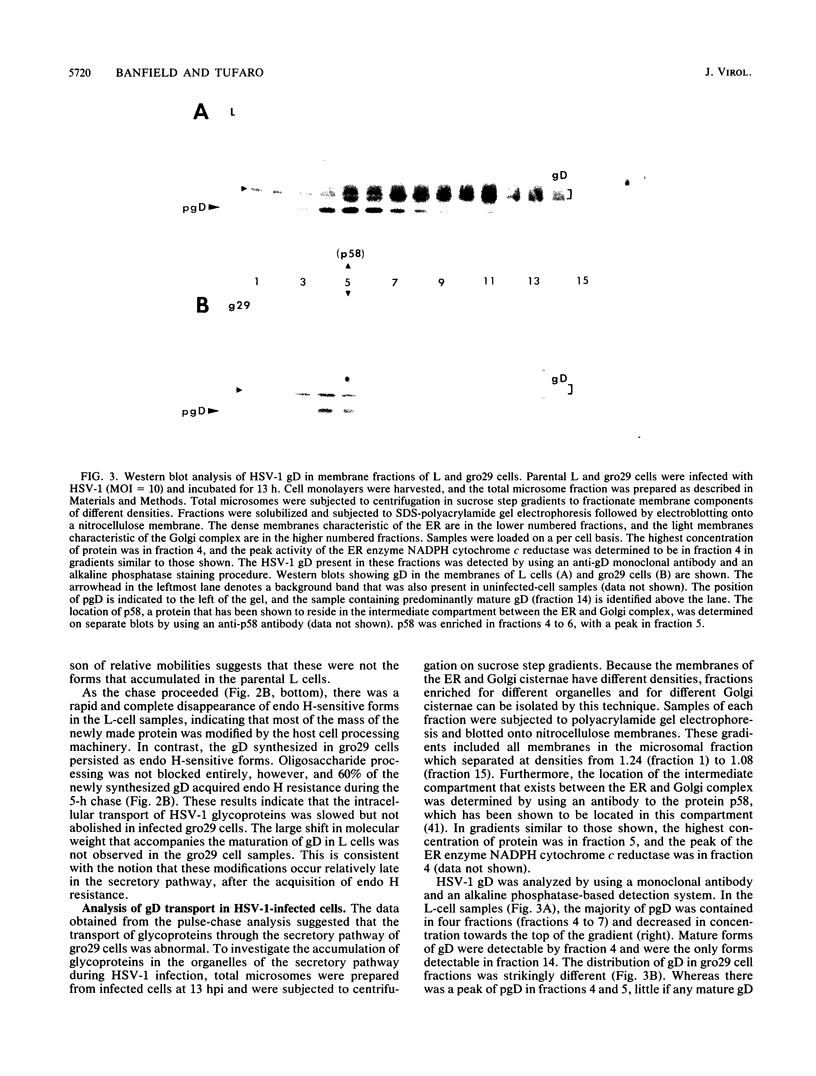
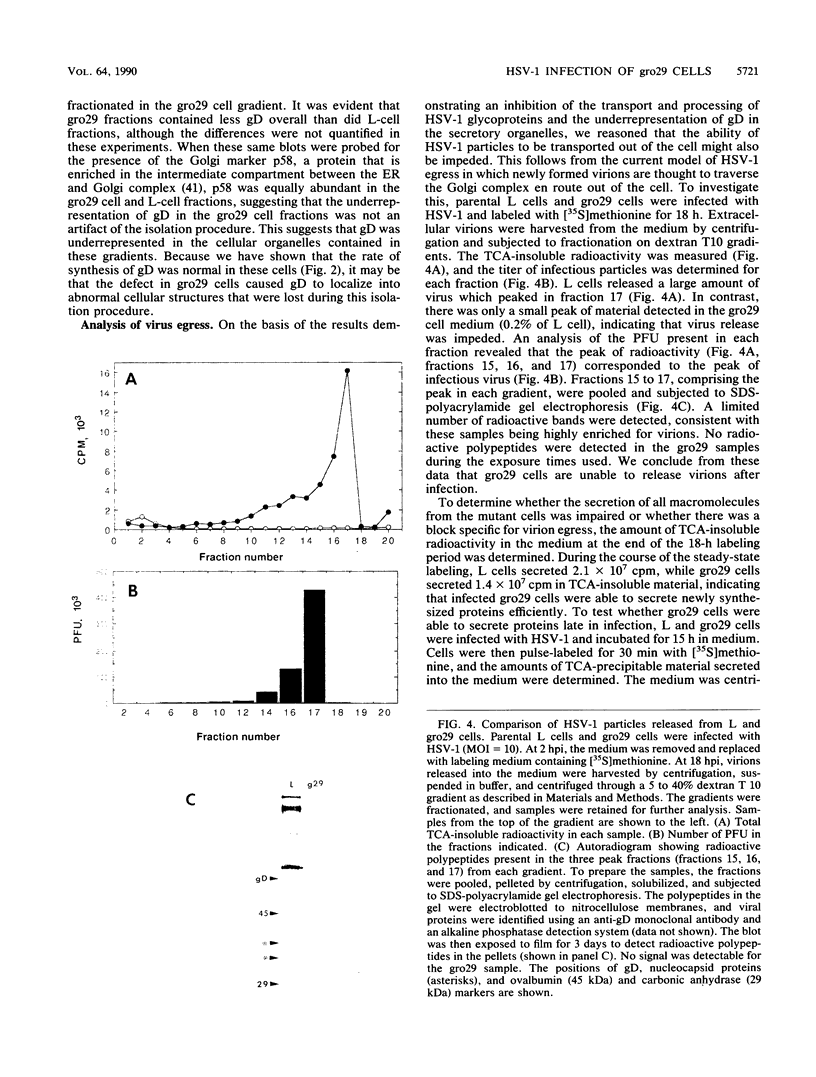
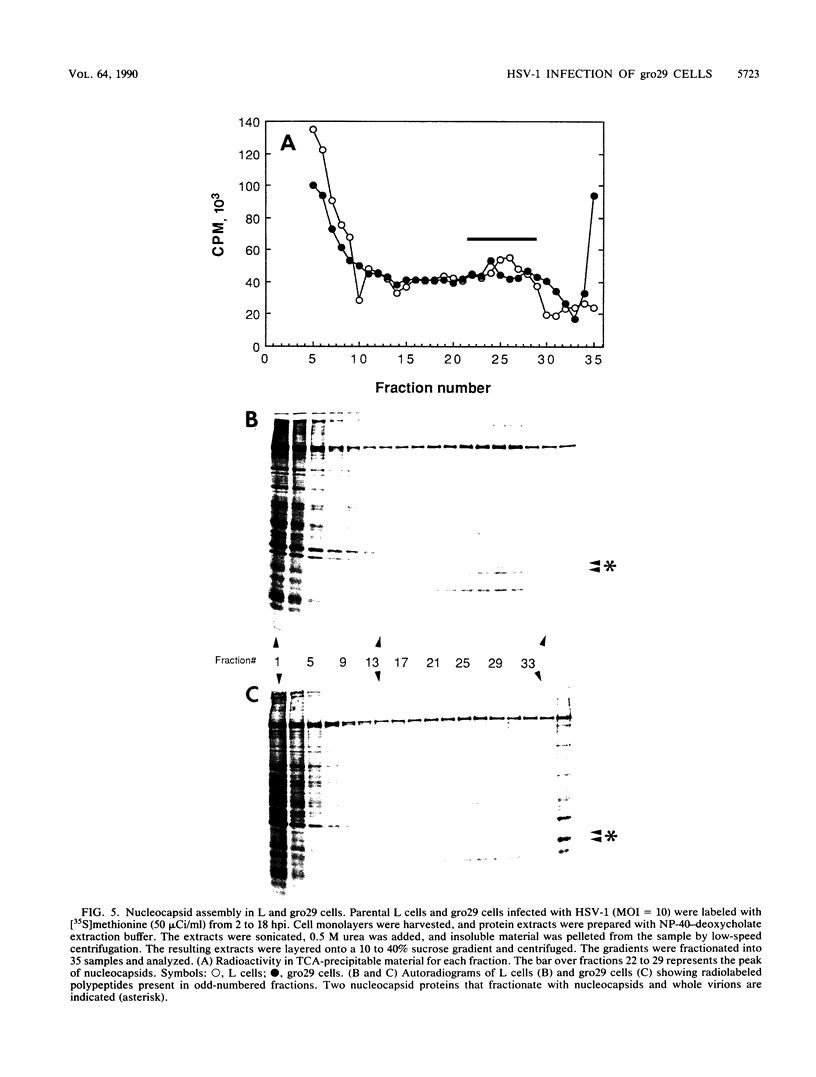
The mouse L-cell mutant gro29 was selected for its ability to survive infection by herpes simplex virus type 1 (HSV-1) and is defective in the propagation of HSV-1 and vesicular stomatitis virus (F. Tufaro, M. D. Snider, and S. L. McKnight, J. Cell Biol. 105:647-657, 1987). In this report, we show that gro29 cells harbor a lesion that inhibits the egress of HSV-1 virions during infection. We also found that HSV-1 glycoprotein D was slow to traverse the secretory pathway en route to the plasma membrane of infected gro29 cells. The movement of glycoproteins was not blocked entirely, however, and immunofluorescence experiments revealed that infected gro29 cells contained roughly 10% of the expected amount of glycoprotein D on their cell surface at 12 h postinfection. Furthermore, nucleocapsids and virions assembled inside the cells during infection, suggesting that the lesion in gro29 cells impinged on a late step in virion maturation. Electron micrographs of infected cells revealed that many of the intracellular virions were contained in irregular cytoplasmic vacuoles, similar to those that accumulate in HSV-1-infected cells treated with the ionophore monensin. We conclude from these results that gro29 harbors a defect that blocks the egress of HSV-1 virions from the infected cell without seriously impeding the flux of individual glycoproteins to the cell surface. We infer that HSV-1 maturation and egress require a host cell component that is either reduced or absent in gro29 cells and that this lesion, although not lethal to the host cell, cannot be tolerated by HSV-1 during its life cycle.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann M., Longnecker R., Roizman B., Pereira L. Identification, properties, and gene location of a novel glycoprotein specified by herpes simplex virus 1. Virology. 1986 Apr 15;150(1):207–220. doi: 10.1016/0042-6822(86)90280-1. [DOI] [PubMed] [Google Scholar]
- Balch W. E., Dunphy W. G., Braell W. A., Rothman J. E. Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell. 1984 Dec;39(2 Pt 1):405–416. doi: 10.1016/0092-8674(84)90019-9. [DOI] [PubMed] [Google Scholar]
- Balch W. E., Wagner K. R., Keller D. S. Reconstitution of transport of vesicular stomatitis virus G protein from the endoplasmic reticulum to the Golgi complex using a cell-free system. J Cell Biol. 1987 Mar;104(3):749–760. doi: 10.1083/jcb.104.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers C. J., Keller D. S., Balch W. E. Semi-intact cells permeable to macromolecules: use in reconstitution of protein transport from the endoplasmic reticulum to the Golgi complex. Cell. 1987 Aug 14;50(4):523–534. doi: 10.1016/0092-8674(87)90025-0. [DOI] [PubMed] [Google Scholar]
- Braell W. A., Balch W. E., Dobbertin D. C., Rothman J. E. The glycoprotein that is transported between successive compartments of the Golgi in a cell-free system resides in stacks of cisternae. Cell. 1984 Dec;39(3 Pt 2):511–524. doi: 10.1016/0092-8674(84)90458-6. [DOI] [PubMed] [Google Scholar]
- Buckmaster E. A., Gompels U., Minson A. Characterisation and physical mapping of an HSV-1 glycoprotein of approximately 115 X 10(3) molecular weight. Virology. 1984 Dec;139(2):408–413. doi: 10.1016/0042-6822(84)90387-8. [DOI] [PubMed] [Google Scholar]
- Burke B., Matlin K., Bause E., Legler G., Peyrieras N., Ploegh H. Inhibition of N-linked oligosaccharide trimming does not interfere with surface expression of certain integral membrane proteins. EMBO J. 1984 Mar;3(3):551–556. doi: 10.1002/j.1460-2075.1984.tb01845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzik D. J., Fox B. A., DeLuca N. A., Person S. Nucleotide sequence specifying the glycoprotein gene, gB, of herpes simplex virus type 1. Virology. 1984 Mar;133(2):301–314. doi: 10.1016/0042-6822(84)90397-0. [DOI] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Lombardo M. T., Foà-Tomasi L., Avitabile E., Serafini-Cessi F. Individual herpes simplex virus 1 glycoproteins display characteristic rates of maturation from precursor to mature form both in infected cells and in cells that constitutively express the glycoproteins. Virus Res. 1988 Apr;10(1):29–40. doi: 10.1016/0168-1702(88)90055-x. [DOI] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Poletti L., Dall'Olio F., Serafini-Cessi F. Infectivity and glycoprotein processing of herpes simplex virus type 1 grown in a ricin-resistant cell line deficient in N-acetylglucosaminyl transferase I. J Virol. 1982 Sep;43(3):1061–1071. doi: 10.1128/jvi.43.3.1061-1071.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Long D., Matthews J. T., May M., Eisenberg R. Glycopeptides of the type-common glycoprotein gD of herpes simplex virus types 1 and 2. J Virol. 1983 Jun;46(3):679–689. doi: 10.1128/jvi.46.3.679-689.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton T., Courtney R. J. Virus-specific glycoproteins associated with the nuclear fraction of herpes simplex virus type 1-infected cells. J Virol. 1984 Feb;49(2):594–597. doi: 10.1128/jvi.49.2.594-597.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington R. W., Moss L. H., 3rd Herpesvirus envelopment. J Virol. 1968 Jan;2(1):48–55. doi: 10.1128/jvi.2.1.48-55.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb C., Baenziger J., Kornfeld S. Deficient uridine diphosphate-N-acetylglucosamine:glycoprotein N-acetylglucosaminyltransferase activity in a clone of Chinese hamster ovary cells with altered surface glycoproteins. J Biol Chem. 1975 May 10;250(9):3303–3309. [PubMed] [Google Scholar]
- Gottlieb C., Kornfeld S., Schlesinger S. Restricted replication of two alphaviruses in ricin-resistant mouse L cells with altered glycosyltransferase activities. J Virol. 1979 Jan;29(1):344–351. doi: 10.1128/jvi.29.1.344-351.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing J., Gething M. J., Sambrook J. Addition of truncated oligosaccharides to influenza virus hemagglutinin results in its temperature-conditional cell-surface expression. J Cell Biol. 1989 Feb;108(2):355–365. doi: 10.1083/jcb.108.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing J., Hunter E., Rodgers L., Gething M. J., Sambrook J. Isolation of Chinese hamster ovary cell lines temperature conditional for the cell-surface expression of integral membrane glycoproteins. J Cell Biol. 1989 Feb;108(2):339–353. doi: 10.1083/jcb.108.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt L. A. Altered synthesis and processing of oligosaccharides of vesicular stomatitis virus glycoprotein in different lectin-resistant Chinese hamster ovary cell lines. J Virol. 1980 Aug;35(2):362–370. doi: 10.1128/jvi.35.2.362-370.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Schlesinger M. J. Vesicular stomatitis virus and sindbis virus glycoprotein transport to the cell surface is inhibited by ionophores. Virology. 1980 Jun;103(2):407–424. doi: 10.1016/0042-6822(80)90200-7. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J Virol. 1982 Sep;43(3):1102–1112. doi: 10.1128/jvi.43.3.1102-1112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. O-linked oligosaccharides are acquired by herpes simplex virus glycoproteins in the Golgi apparatus. Cell. 1983 Mar;32(3):987–997. doi: 10.1016/0092-8674(83)90083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones F., Grose C. Role of cytoplasmic vacuoles in varicella-zoster virus glycoprotein trafficking and virion envelopment. J Virol. 1988 Aug;62(8):2701–2711. doi: 10.1128/jvi.62.8.2701-2711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley D. M., Kozarsky K. F., Hobbie L., Krieger M. Reversible defects in O-linked glycosylation and LDL receptor expression in a UDP-Gal/UDP-GalNAc 4-epimerase deficient mutant. Cell. 1986 Mar 14;44(5):749–759. doi: 10.1016/0092-8674(86)90841-x. [DOI] [PubMed] [Google Scholar]
- Kingsley D. M., Kozarsky K. F., Segal M., Krieger M. Three types of low density lipoprotein receptor-deficient mutant have pleiotropic defects in the synthesis of N-linked, O-linked, and lipid-linked carbohydrate chains. J Cell Biol. 1986 May;102(5):1576–1585. doi: 10.1083/jcb.102.5.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley D. M., Krieger M. Receptor-mediated endocytosis of low density lipoprotein: somatic cell mutants define multiple genes required for expression of surface-receptor activity. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5454–5458. doi: 10.1073/pnas.81.17.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kuismanen E., Bång B., Hurme M., Pettersson R. F. Uukuniemi virus maturation: immunofluorescence microscopy with monoclonal glycoprotein-specific antibodies. J Virol. 1984 Jul;51(1):137–146. doi: 10.1128/jvi.51.1.137-146.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuismanen E., Hedman K., Saraste J., Pettersson R. F. Uukuniemi virus maturation: accumulation of virus particles and viral antigens in the Golgi complex. Mol Cell Biol. 1982 Nov;2(11):1444–1458. doi: 10.1128/mcb.2.11.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuismanen E., Saraste J., Pettersson R. F. Effect of monensin on the assembly of Uukuniemi virus in the Golgi complex. J Virol. 1985 Sep;55(3):813–822. doi: 10.1128/jvi.55.3.813-822.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker R., Chatterjee S., Whitley R. J., Roizman B. Identification of a herpes simplex virus 1 glycoprotein gene within a gene cluster dispensable for growth in cell culture. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4303–4307. doi: 10.1073/pnas.84.12.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN C., ROSE H. M., HOLDEN M., JONES E. P. Electron microscopic observations on the development of herpes simplex virus. J Exp Med. 1959 Oct 1;110:643–656. doi: 10.1084/jem.110.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J. T., Cohen G. H., Eisenberg R. J. Synthesis and processing of glycoprotein D of herpes simplex virus types 1 and 2 in an in vitro system. J Virol. 1983 Nov;48(2):521–533. doi: 10.1128/jvi.48.2.521-533.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano A., Nishijima M., Maeda M., Akamatsu Y. A temperature-sensitive Chinese hamster ovary cell mutant pleiotropically defective in protein export. Biochim Biophys Acta. 1985 Jun 30;845(3):324–332. doi: 10.1016/0167-4889(85)90195-8. [DOI] [PubMed] [Google Scholar]
- Nii S., Morgan C., Rose H. M. Electron microscopy of herpes simplex virus. II. Sequence of development. J Virol. 1968 May;2(5):517–536. doi: 10.1128/jvi.2.5.517-536.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Richman D. D., Buckmaster A., Bell S., Hodgman C., Minson A. C. Identification of a new glycoprotein of herpes simplex virus type 1 and genetic mapping of the gene that codes for it. J Virol. 1986 Feb;57(2):647–655. doi: 10.1128/jvi.57.2.647-655.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rixon F. J., Davison M. D., Davison A. J. Identification of the genes encoding two capsid proteins of herpes simplex virus type 1 by direct amino acid sequencing. J Gen Virol. 1990 May;71(Pt 5):1211–1214. doi: 10.1099/0022-1317-71-5-1211. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., Dubois-Dalcq M. Intramembrane changes occurring during maturation of herpes simplex virus type 1: freeze-fracture study. J Virol. 1978 May;26(2):435–447. doi: 10.1128/jvi.26.2.435-447.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J., Palade G. E., Farquhar M. G. Antibodies to rat pancreas Golgi subfractions: identification of a 58-kD cis-Golgi protein. J Cell Biol. 1987 Nov;105(5):2021–2029. doi: 10.1083/jcb.105.5.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J., Palade G. E., Farquhar M. G. Temperature-sensitive steps in the transport of secretory proteins through the Golgi complex in exocrine pancreatic cells. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6425–6429. doi: 10.1073/pnas.83.17.6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini-Cessi F., Campadelli-Fiume G. Studies on benzhydrazone, a specific inhibitor of herpesvirus glycoprotein synthesis. Size distribution of glycopeptides and endo-beta-N-acetylglucosaminidase-H treatment. Arch Virol. 1981;70(4):331–343. doi: 10.1007/BF01320248. [DOI] [PubMed] [Google Scholar]
- Serafini-Cessi F., Dall'Olio F., Malagolini N., Pereira L., Campadelli-Fiume G. Comparative study on O-linked oligosaccharides of glycoprotein D of herpes simplex virus types 1 and 2. J Gen Virol. 1988 Apr;69(Pt 4):869–877. doi: 10.1099/0022-1317-69-4-869. [DOI] [PubMed] [Google Scholar]
- Serafini-Cessi F., Dall'Olio F., Scannavini M., Campadelli-Fiume G. Processing of herpes simplex virus-1 glycans in cells defective in glycosyl transferases of the Golgi system: relationship to cell fusion and virion egress. Virology. 1983 Nov;131(1):59–70. doi: 10.1016/0042-6822(83)90533-0. [DOI] [PubMed] [Google Scholar]
- Stanley P. Glycosylation mutants of animal cells. Annu Rev Genet. 1984;18:525–552. doi: 10.1146/annurev.ge.18.120184.002521. [DOI] [PubMed] [Google Scholar]
- Tooze J., Tooze S. A. Infection of AtT20 murine pituitary tumour cells by mouse hepatitis virus strain A59: virus budding is restricted to the Golgi region. Eur J Cell Biol. 1985 May;37:203–212. [PubMed] [Google Scholar]
- Tooze J., Tooze S., Warren G. Replication of coronavirus MHV-A59 in sac- cells: determination of the first site of budding of progeny virions. Eur J Cell Biol. 1984 Mar;33(2):281–293. [PubMed] [Google Scholar]
- Trowbridge I. S., Hyman R., Mazauskas C. The synthesis and properties of T25 blycoprotein in Thy-1-negative mutant lymphoma cells. Cell. 1978 May;14(1):21–32. doi: 10.1016/0092-8674(78)90297-0. [DOI] [PubMed] [Google Scholar]
- Tufaro F., Snider M. D., McKnight S. L. Identification and characterization of a mouse cell mutant defective in the intracellular transport of glycoproteins. J Cell Biol. 1987 Aug;105(2):647–657. doi: 10.1083/jcb.105.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vischer P., Hughes R. C. Glycosyl transferases of baby-hamster-kidney (BHK) cells and ricin-resistant mutants. N-glycan biosynthesis. Eur J Biochem. 1981 Jul;117(2):275–284. doi: 10.1111/j.1432-1033.1981.tb06334.x. [DOI] [PubMed] [Google Scholar]
- Weiss M., Horzinek M. C. Morphogenesis of Berne virus (proposed family Toroviridae). J Gen Virol. 1986 Jul;67(Pt 7):1305–1314. doi: 10.1099/0022-1317-67-7-1305. [DOI] [PubMed] [Google Scholar]
- Wenske E. A., Bratton M. W., Courtney R. J. Endo-beta-N-acetylglucosaminidase H sensitivity of precursors to herpes simplex virus type 1 glycoproteins gB and gC. J Virol. 1982 Oct;44(1):241–248. doi: 10.1128/jvi.44.1.241-248.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]