Abstract
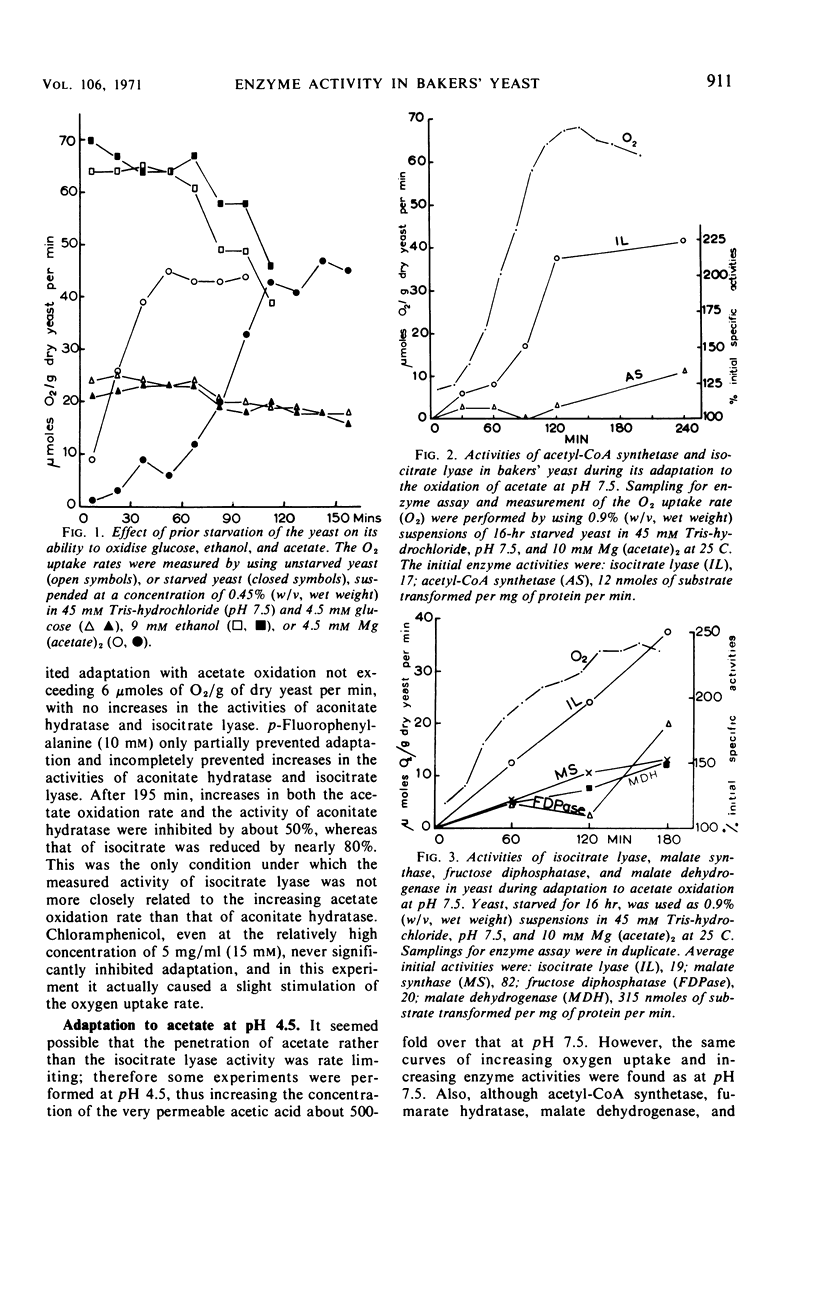
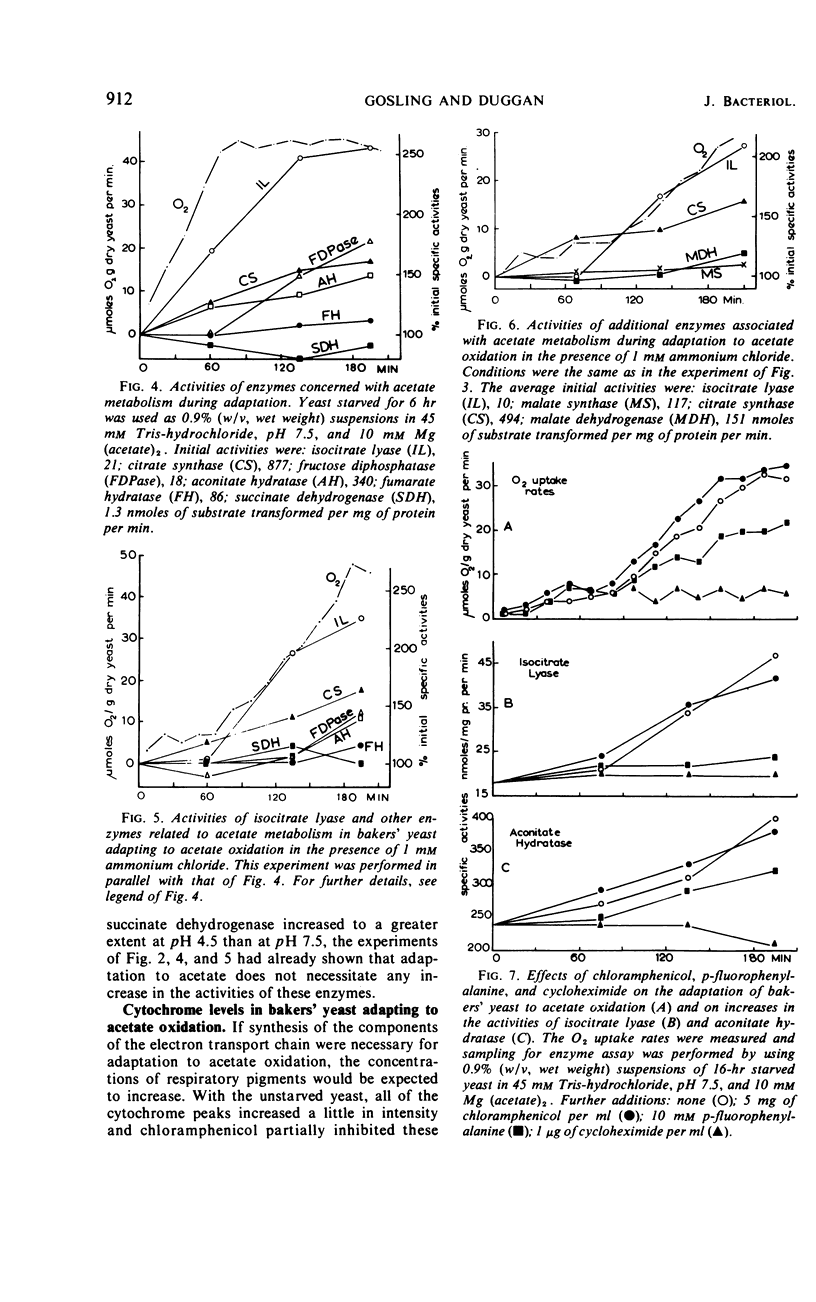
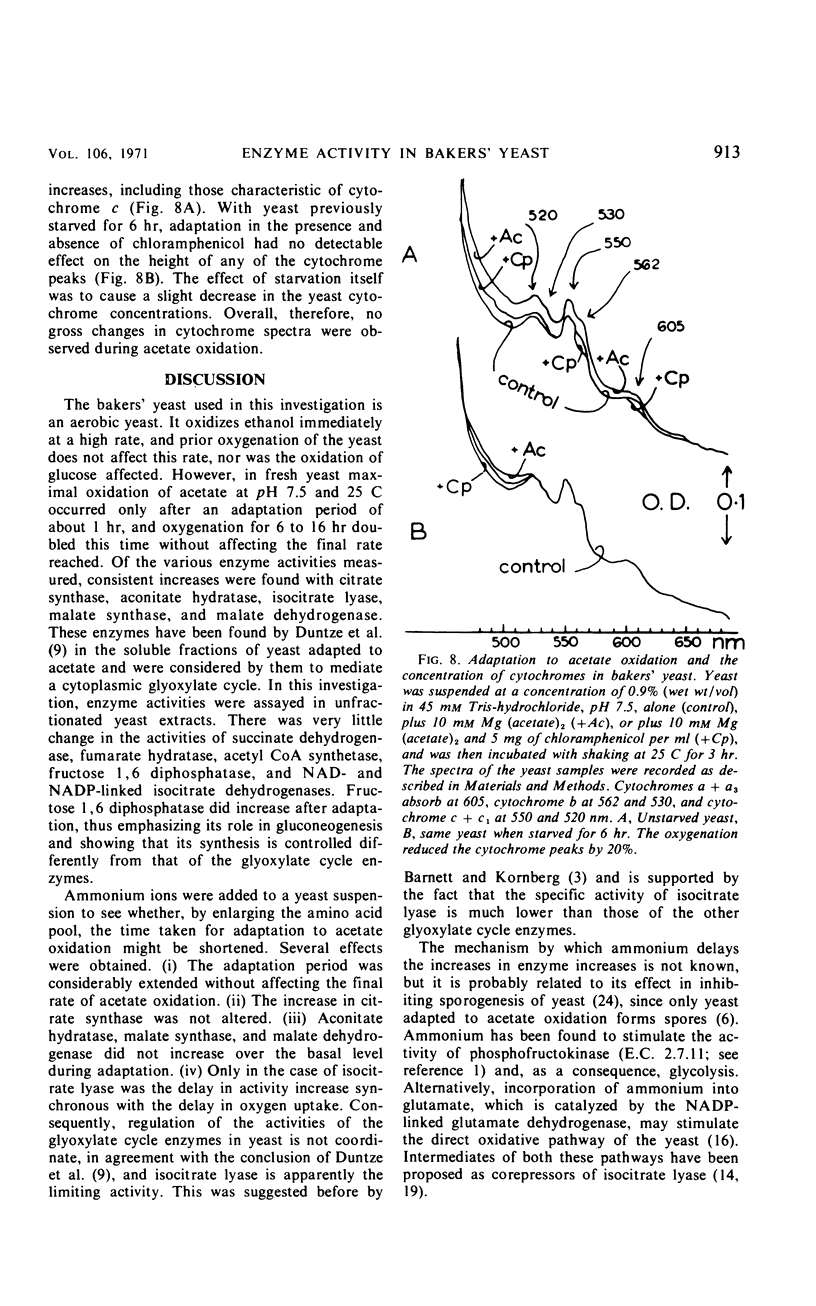
Bakers' yeast oxidizes acetate at a high rate only after an adaptation period during which the capacity of the glyoxylate cycle is found to increase. There was apparently no necessity for the activity of acetyl-coenzyme A synthetase, the capacity of the tricarboxylic acid cycle, or the concentrations of the cytochromes to increase for this adaptation to occur. Elevation of fructose 1,6 diphosphatase occurred only when acetate oxidation was nearly maximal. Cycloheximide almost completely inhibited adaptation as well as increases in the activities of isocitrate lyase and aconitate hydratase, the only enzymes assayed. p-Fluorophenylalanine was partially effective and chloramphenicol did not inhibit at all. The presence of ammonium, which considerably delayed adaptation of the yeast to acetate oxidation, inhibited the increases in the activities of the glyoxylate cycle enzymes to different degrees, demonstrating noncoordinate control of these enzymes. Under the various conditions, the only enzyme activity increase consistently related to the rising oxygen uptake rate was that of isocitrate lyase which apparently limited the activity of the cycle.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atzpodien W., Bode H. Purification and regulatory properties of ATP-sensitive phosphofructokinase from yeast. Eur J Biochem. 1970 Jan;12(1):126–132. doi: 10.1111/j.1432-1033.1970.tb00829.x. [DOI] [PubMed] [Google Scholar]
- Avers C. J., Federman M. The occurrence in yeast of cytoplasmic granules which resemble microbodies. J Cell Biol. 1968 May;37(2):555–559. doi: 10.1083/jcb.37.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNETT J. A., KORNBERG H. L. The utilization by yeasts of acids of the tricarboxylic acid cycle. J Gen Microbiol. 1960 Aug;23:65–82. doi: 10.1099/00221287-23-1-65. [DOI] [PubMed] [Google Scholar]
- Breidenbach R. W., Beevers H. Association of the glyoxylate cycle enzymes in a novel subcellular particle from castor bean endosperm. Biochem Biophys Res Commun. 1967 May 25;27(4):462–469. doi: 10.1016/s0006-291x(67)80007-x. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D., Linnane A. W. In vivo differentiation of yeast cytoplasmic and mitochondrial protein synthesis with antibiotics. Biochem Biophys Res Commun. 1966 Oct 5;25(1):8–13. doi: 10.1016/0006-291x(66)90631-0. [DOI] [PubMed] [Google Scholar]
- DUGGAN P. F. ACETATE AND ETHANOL OXIDATION BY YEAST. ASPECTS OF THE METABOLISM OF ACETATE AND ETHANOL IN YEAST. Ir J Med Sci. 1964 Jan;457:19–30. doi: 10.1007/BF02953857. [DOI] [PubMed] [Google Scholar]
- Duntze W., Neumann D., Gancedo J. M., Atzpodien W., Holzer H. Studies on the regulation and localization of the glyoxylate cycle enzymes in Saccharomyces cerevisiae. Eur J Biochem. 1969 Aug;10(1):83–89. doi: 10.1111/j.1432-1033.1969.tb00658.x. [DOI] [PubMed] [Google Scholar]
- EATON N. R., KLEIN H. P. The oxidation of glucose and acetate by Saccharomyces cerevisiae. J Bacteriol. 1954 Jul;68(1):110–116. doi: 10.1128/jb.68.1.110-116.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell R. B., Fincham J. R. Acetate-nonutilizing mutants of Neurospora rassa. II. Biochemical deficiencies and the roles of certain enzymes. J Bacteriol. 1968 Mar;95(3):1063–1068. doi: 10.1128/jb.95.3.1063-1068.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosling J. P., Duggan P. F. Adaptation of baker's yeast to the oxidation of acetate. Biochem J. 1968 Nov;110(2):19P–20P. doi: 10.1042/bj1100019pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosling J. P., Duggan P. F. The oxidation of acetate, ethanol and pyruvate by baker's yeast. Biochem J. 1969 Nov;115(3):8P–9P. doi: 10.1042/bj1150008pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerritore A., Hanozet G. M., Cocucci M. C. Regulation of isocitrate lyase level in yeast growing on external carbon sources or on lipid reserves. Experientia. 1969 Feb 15;25(2):131–132. doi: 10.1007/BF01899079. [DOI] [PubMed] [Google Scholar]
- HALVORSON H. O., SPIEGELMAN S. The effect of free amino acid pool levels on the induced synthesis of enzymes. J Bacteriol. 1953 May;65(5):496–504. doi: 10.1128/jb.65.5.496-504.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLZER H., WITT I. [Acceleration of the oxidative pentose phosphate cycle in yeast cells by ammonium salts]. Biochim Biophys Acta. 1960 Feb 12;38:163–164. doi: 10.1016/0006-3002(60)91214-2. [DOI] [PubMed] [Google Scholar]
- KEMPNER E. S., COWIE D. B. Metabolic pools and the utilization of amino acid analogs for protein synthesis. Biochim Biophys Acta. 1960 Aug 26;42:401–408. doi: 10.1016/0006-3002(60)90817-9. [DOI] [PubMed] [Google Scholar]
- KORNBERG H. L., KREBS H. A. Synthesis of cell constituents from C2-units by a modified tricarboxylic acid cycle. Nature. 1957 May 18;179(4568):988–991. doi: 10.1038/179988a0. [DOI] [PubMed] [Google Scholar]
- Klein H. P., Jahnke L. Cellular localization of acetyl-coenzyme A synthetase in yeast. J Bacteriol. 1968 Nov;96(5):1632–1639. doi: 10.1128/jb.96.5.1632-1639.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg H. L. The role and control of the glyoxylate cycle in Escherichia coli. Biochem J. 1966 Apr;99(1):1–11. doi: 10.1042/bj0990001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968 May;37(2):482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Hogg J. F., De Duve C. Distribution of tricarboxylic acid cycle enzymes and glyoxylate cycle enzymes between mitochondria and peroxisomes in Tetrahymena pyriformis. J Biol Chem. 1968 Oct 25;243(20):5385–5395. [PubMed] [Google Scholar]
- Polakis E. S., Bartley W. Changes in the enzyme activities of Saccharomyces cerevisiae during aerobic growth on different carbon sources. Biochem J. 1965 Oct;97(1):284–297. doi: 10.1042/bj0970284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis E. S., Bartley W., Meek G. A. Changes in the structure and enzyme activity of Saccharomyces cerevisiae in response to changes in the environment. Biochem J. 1964 Feb;90(2):369–374. doi: 10.1042/bj0900369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- Witt I., Kronau R., Holzer H. Repression von Alkoholdehydrogenase, Malatdehydrogenase, Isocitratlyase und Malatsynthase in Hefe durch Glucose. Biochim Biophys Acta. 1966 Jun 15;118(3):522–537. [PubMed] [Google Scholar]


