Abstract
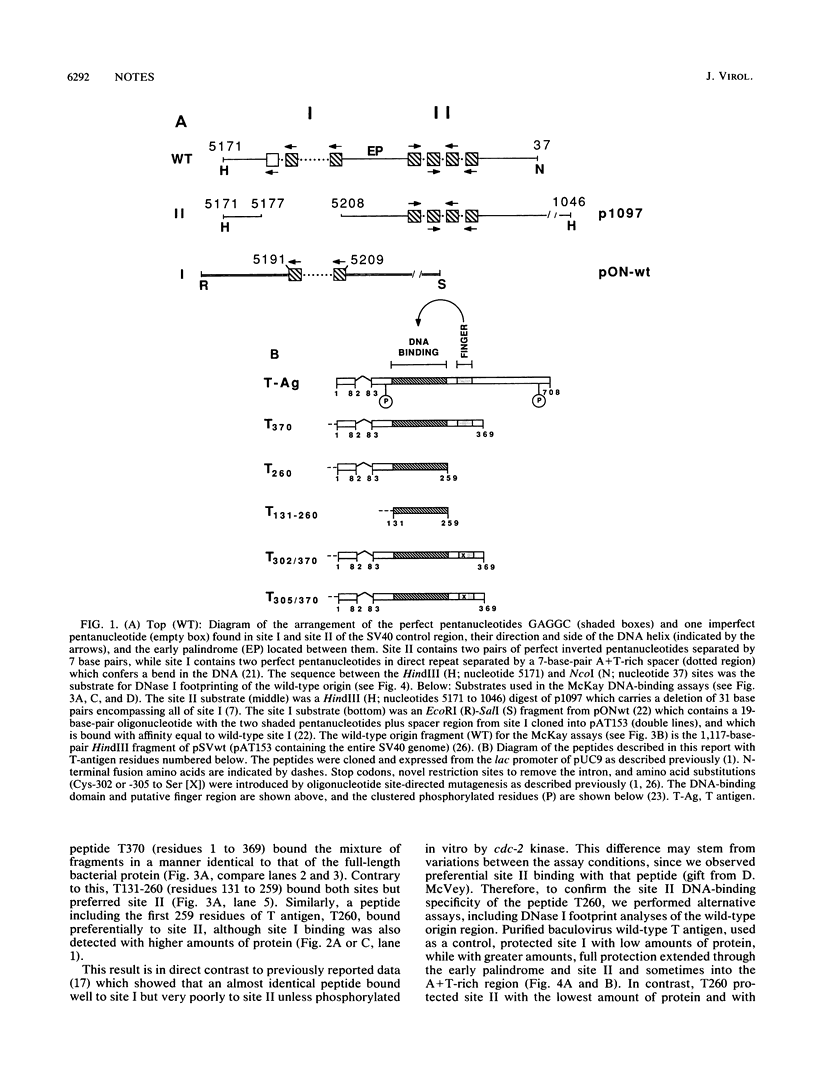
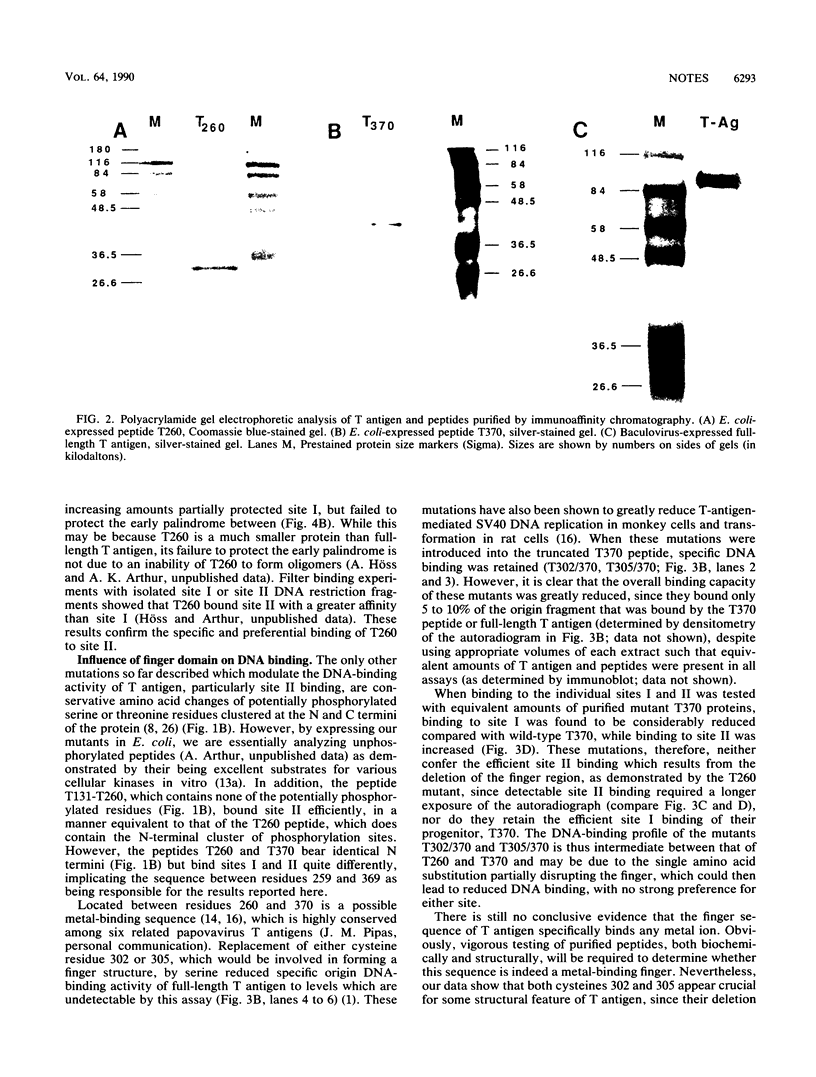
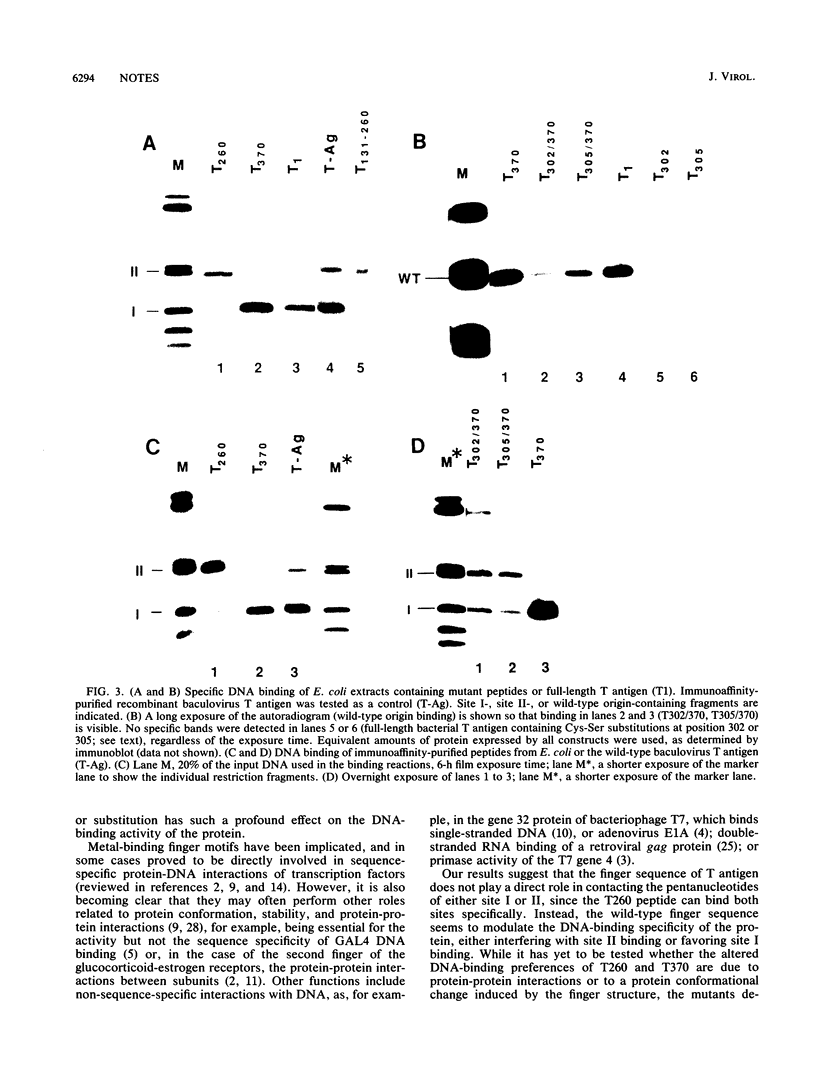
The specificity and regulation of protein-DNA interactions play a crucial role in all aspects of communication between genotype and phenotype in a cell. The large T antigen of simian virus 40 binds to identical, yet quite differently arranged, pentanucleotide motifs in the simian virus 40 control region, sites I and II. Wild-type T antigen preferentially binds site I. We demonstrate that a bacterial peptide encoding residues 1 to 259 (T260) includes the essential amino acids required for binding to both DNA sites but predominantly binds site II. However, a longer peptide (residues 1 to 369; T370) binds almost exclusively to site I. Thus, the addition of amino acids 260 to 369 to the T260 peptide results in the loss of site II binding. This region includes a single putative metal-binding region, and mutation of T370 at either conserved cysteine of the finger results in equal but inefficient binding to both sites. While no metal binding has been shown to be directly associated with this sequence, these results suggest a novel, perhaps structural, function for such a finger motif, since this domain of T antigen appears to play a crucial role in modulating the DNA-binding behavior of T-antigen peptides.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur A. K., Höss A., Fanning E. Expression of simian virus 40 T antigen in Escherichia coli: localization of T-antigen origin DNA-binding domain to within 129 amino acids. J Virol. 1988 Jun;62(6):1999–2006. doi: 10.1128/jvi.62.6.1999-2006.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg J. M. DNA binding specificity of steroid receptors. Cell. 1989 Jun 30;57(7):1065–1068. doi: 10.1016/0092-8674(89)90042-1. [DOI] [PubMed] [Google Scholar]
- Bernstein J. A., Richardson C. C. A 7-kDa region of the bacteriophage T7 gene 4 protein is required for primase but not for helicase activity. Proc Natl Acad Sci U S A. 1988 Jan;85(2):396–400. doi: 10.1073/pnas.85.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee P. K., Bruner M., Flint S. J., Harter M. L. DNA-binding properties of an adenovirus 289R E1A protein. EMBO J. 1988 Mar;7(3):835–841. doi: 10.1002/j.1460-2075.1988.tb02882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corton J. C., Johnston S. A. Altering DNA-binding specificity of GAL4 requires sequences adjacent to the zinc finger. Nature. 1989 Aug 31;340(6236):724–727. doi: 10.1038/340724a0. [DOI] [PubMed] [Google Scholar]
- DiMaio D., Nathans D. Regulatory mutants of simian virus 40. Effect of mutations at a T antigen binding site on DNA replication and expression of viral genes. J Mol Biol. 1982 Apr 15;156(3):531–548. doi: 10.1016/0022-2836(82)90265-0. [DOI] [PubMed] [Google Scholar]
- Fanning E., Schneider J., Arthur A., Höss A., Moarefi I., Modrow S. Structure and function of SV 40 large T antigen: communication between functional domains. Curr Top Microbiol Immunol. 1989;144:9–19. doi: 10.1007/978-3-642-74578-2_2. [DOI] [PubMed] [Google Scholar]
- Frankel A. D., Pabo C. O. Fingering too many proteins. Cell. 1988 Jun 3;53(5):675–675. doi: 10.1016/0092-8674(88)90083-9. [DOI] [PubMed] [Google Scholar]
- Giedroc D. P., Keating K. M., Williams K. R., Coleman J. E. The function of zinc in gene 32 protein from T4. Biochemistry. 1987 Aug 25;26(17):5251–5259. doi: 10.1021/bi00391a007. [DOI] [PubMed] [Google Scholar]
- Green S., Kumar V., Theulaz I., Wahli W., Chambon P. The N-terminal DNA-binding 'zinc finger' of the oestrogen and glucocorticoid receptors determines target gene specificity. EMBO J. 1988 Oct;7(10):3037–3044. doi: 10.1002/j.1460-2075.1988.tb03168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höss A., Moarefi I., Scheidtmann K. H., Cisek L. J., Corden J. L., Dornreiter I., Arthur A. K., Fanning E. Altered phosphorylation pattern of simian virus 40 T antigen expressed in insect cells by using a baculovirus vector. J Virol. 1990 Oct;64(10):4799–4807. doi: 10.1128/jvi.64.10.4799-4807.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanford R. E. Expression of simian virus 40 T antigen in insect cells using a baculovirus expression vector. Virology. 1988 Nov;167(1):72–81. doi: 10.1016/0042-6822(88)90055-4. [DOI] [PubMed] [Google Scholar]
- Loeber G., Parsons R., Tegtmeyer P. The zinc finger region of simian virus 40 large T antigen. J Virol. 1989 Jan;63(1):94–100. doi: 10.1128/jvi.63.1.94-100.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey D., Brizuela L., Mohr I., Marshak D. R., Gluzman Y., Beach D. Phosphorylation of large tumour antigen by cdc2 stimulates SV40 DNA replication. Nature. 1989 Oct 12;341(6242):503–507. doi: 10.1038/341503a0. [DOI] [PubMed] [Google Scholar]
- McVey D., Strauss M., Gluzman Y. Properties of the DNA-binding domain of the simian virus 40 large T antigen. Mol Cell Biol. 1989 Dec;9(12):5525–5536. doi: 10.1128/mcb.9.12.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr I. J., Gluzman Y., Fairman M. P., Strauss M., McVey D., Stillman B., Gerard R. D. Production of simian virus 40 large tumor antigen in bacteria: altered DNA-binding specificity and dna-replication activity of underphosphorylated large tumor antigen. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6479–6483. doi: 10.1073/pnas.86.17.6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C. I., Weiner B., Bikel I., Piwnica-Worms H., Bradley M. K., Livingston D. M. Purification and functional properties of simian virus 40 large and small T antigens overproduced in insect cells. J Virol. 1988 Aug;62(8):2951–2959. doi: 10.1128/jvi.62.8.2951-2959.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder K., Silver S., DeLucia A. L., Fanning E., Tegtmeyer P. An altered DNA conformation in origin region I is a determinant for the binding of SV40 large T antigen. Cell. 1986 Mar 14;44(5):719–725. doi: 10.1016/0092-8674(86)90838-x. [DOI] [PubMed] [Google Scholar]
- Ryder K., Vakalopoulou E., Mertz R., Mastrangelo I., Hough P., Tegtmeyer P., Fanning E. Seventeen base pairs of region I encode a novel tripartite binding signal for SV40 T antigen. Cell. 1985 Sep;42(2):539–548. doi: 10.1016/0092-8674(85)90111-4. [DOI] [PubMed] [Google Scholar]
- Scheidtmann K. H., Echle B., Walter G. Simian virus 40 large T antigen is phosphorylated at multiple sites clustered in two separate regions. J Virol. 1982 Oct;44(1):116–133. doi: 10.1128/jvi.44.1.116-133.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Hardung M., Echle B., Walter G. DNA-binding activity of simian virus 40 large T antigen correlates with a distinct phosphorylation state. J Virol. 1984 Apr;50(1):1–12. doi: 10.1128/jvi.50.1.1-12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff L. A., Nibert M. L., Fields B. N. Characterization of a zinc blotting technique: evidence that a retroviral gag protein binds zinc. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4195–4199. doi: 10.1073/pnas.85.12.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J., Fanning E. Mutations in the phosphorylation sites of simian virus 40 (SV40) T antigen alter its origin DNA-binding specificity for sites I or II and affect SV40 DNA replication activity. J Virol. 1988 May;62(5):1598–1605. doi: 10.1128/jvi.62.5.1598-1605.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. Initiation of eukaryotic DNA replication in vitro. Annu Rev Cell Biol. 1989;5:197–245. doi: 10.1146/annurev.cb.05.110189.001213. [DOI] [PubMed] [Google Scholar]
- Struhl K. Helix-turn-helix, zinc-finger, and leucine-zipper motifs for eukaryotic transcriptional regulatory proteins. Trends Biochem Sci. 1989 Apr;14(4):137–140. doi: 10.1016/0968-0004(89)90145-X. [DOI] [PubMed] [Google Scholar]